Blood
Blood Vessels As Guides: How Dendritic Cells of the Immune System Form 3D Networks
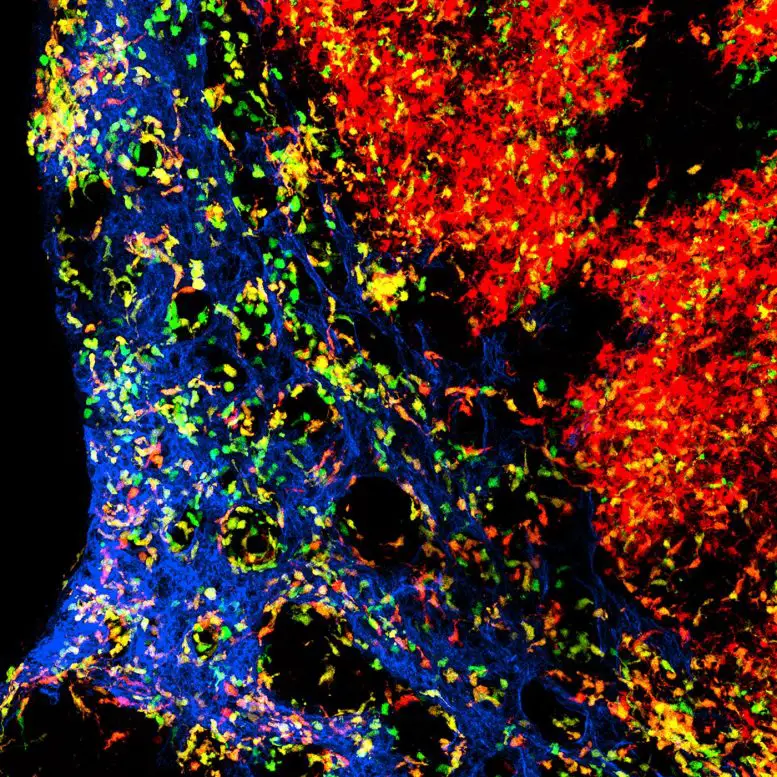
The figure shows the complex organization of dendritic cells in the lymph node. Blood vessels are shown in blue. The cells in green are young dendritic cells whereas the dendritic cells in red are a few days older and have already migrated. The dendritic cells in orange are intermediate in age. Credit: Dr. Milas Ugur / University of Würzburg
Researchers have discovered how short-lived dendritic cells of the immune system form three-dimensional networks, using blood vessels as guides. These networks, regulated by local cytokines, play a crucial role in immune defense and hold high prognostic value for tumor diseases. Further study aims to see if these principles apply universally across tissues and in humans.
The Role of the Immune System and Immune Cells
The cells of the immune system circulate mainly in the blood and migrate into the body’s tissues after an inflammation. Some types of immune cells, however, are permanently located in the tissues, where they come together to form three-dimensional networks.
How do these networks form and how are they maintained? For the long-lived macrophages (phagocytes), the answer is already known: They settle in so-called niches. These are environments of connective tissue cells that supply the macrophages with nutrients and keep them alive.
A Closer Look at Dendritic Cells
A team led by Professors Georg Gasteiger, Dominic Grün, and Wolfgang Kastenmüller from the Institute of Systems Immunology at Julius-Maximilians-Universität Würzburg (JMU) / Max Planck Research Group has now turned its attention to a related type of immune cells, the so-called dendritic cells.
These immune cells are essential for the control of immune responses because they are at the first line of defense of the immune system: They recognize foreign structures, take them in and process them into a kind of mugshot. They then present the photo to other immune cells and trigger a specific immune response, for example against pathogens or cancer cells.
@media(min-width:0px){#div-gpt-ad-scitechdaily_com-medrectangle-4-0-asloaded{max-width:580px!important;max-height:400px!important}}
Dendritic Cells Migrate Through the Tissue
The special thing about dendritic cells is they only live for about a week and during this time they continuously migrate through the body’s tissues. “In this respect, it was clear that the classic niche concept would not work here,” says Wolfgang Kastenmüller.
The JMU team found a completely new concept for this, according to which three-dimensional cell networks can organize themselves: Dendritic cells orient themselves to the blood vessels and migrate one after the other along their outer wall – similar to children walking in single file. The blood vessels thus determine the three-dimensional arrangement of the cells.
Role of Cytokines in Network Regulation
“We wanted to understand how this process is regulated and how the cells manage to close gaps in their network,” explains Dr. Milas Ugur, a scientist in Professor Kastenmüller’s group. Closing such gaps is important because otherwise the immune defense no longer functions optimally.
As the JMU team reports in the journal Immunity, it is due to a locally acting cytokine, the FLT3 ligand, that the dendritic cells always stay close together during their developmental migration.
@media(min-width:0px){#div-gpt-ad-scitechdaily_com-box-4-0-asloaded{max-width:580px!important;max-height:400px!important}}
The cytokines are continuously and evenly produced locally and consumed by the dendritic cells. If there are gaps in the group, more cytokines are available for the isolated dendritic cells. This surplus speeds them up in their development and movement and helps them to reconnect with the group. When the cells have moved up, they have a little less cytokines available again due to competition from their neighbors. Accordingly, they slow down their developmental speed.
Prognostic Significance for Tumor Diseases
These findings are for example important for cancer therapy: dendritic cells have a high prognostic value for tumor diseases: The higher their abundance in the tumor, the better the prognosis for the patient. This is especially true after immunotherapy.
“Increasing our basic insights on dendritic cell biology will help us to restore the networks of these cells in tumors and thereby tailor optimal therapies in the future” explains Kastenmüller.
Next Steps in the Research
The JMU researchers’ data so far is based on the analysis of lymph nodes from animal models. The team next wants to test whether the same principles of network organization of dendritic cells apply to all tissues and also in humans.
Reference: “Lymph node medulla regulates the spatiotemporal unfolding of resident dendritic cell networks” by Milas Ugur, R. Jacob Labios, Chloe Fenton, Konrad Knöpper, Katarzyna Jobin, Fabian Imdahl, Gosia Golda, Kathrin Hoh, Anika Grafen, Tsuneyasu Kaisho, Antoine-Emmanuel Saliba, Dominic Grün, Georg Gasteiger, Marc Bajénoff and Wolfgang Kastenmüller, 17 July 2023, Immunity.
DOI: 10.1016/j.immuni.2023.06.020
The work described was done in cooperation with researchers from the Würzburg Helmholtz Institute for <span class="glossaryLink" aria-describedby="tt" data-cmtooltip="
” data-gt-translate-attributes=”[{“attribute”:”data-cmtooltip”, “format”:”html”}]”>RNA-based Infection Research (HIRI) and with scientists from France and Japan.
Immunology in Würzburg
Würzburg University Medical Center has distinguished itself as an important research location in the field of immunology and has greatly expanded these competencies in recent years. In numerous institutes and chairs, scientists are working on better understanding the immune system and using it to fight diseases. In doing so, they cooperate closely with other researchers in Germany and worldwide.