Infection
The immune responses of Oreochromis niloticus against Prohemistomum vivax encysted metacercariae infection with the evaluation of different biomarkers stressors
Abstract
This study aimed at evaluating the immunological status of Oreochromis niloticus (O. niloticus); so, a total of 120 O. niloticus were collected from different farms located in Kafr El-Sheikh Governorate in Egypt during the period from January 2021 to January 2022. The fish were surveyed for commonly encysted metacercariae present in different organs such as gills, spleen, liver, kidney, and muscles. The collected encysted metacercariae were of the family Cyathocotylidae (Prohemistomum vivax) with a prevalence of 25%. Different cell-mediated immune responses such as Major histocompatibility class II alpha (MHC-IIα), Toll-like receptor 7 (TLR-7), Interleukin (IL-8), and Clusters of differentiation 4 (CD4) were assessed in different organs such as gills, spleen, liver, kidney, and muscles which revealed an elevation in different genes in infected organs as a reaction from the body against parasitic infection. In addition, the liver enzymes; aspartate aminotransferase (AST), and alanine aminotransferase (ALT), were assessed in the serum of O. niloticus as well as blood glucose, cortisol levels, and lysozyme activity were estimated to record higher levels in the infected fish in comparison with the control non-infected ones.
Introduction
Oreochromis niloticus (O. niloticus) plays a significant commercial role in Egypt, yet wild and cultured tilapia are facing a severe loss because of the attack of various ectoparasites, helminths, and protozoa which cause various degrees of diseases in such fish1. Oreochromis niloticus is the primary freshwater fish species found in Egypt’s Nile River (Nile tilapia). For all Egyptians, it is one of the most common and inexpensive fish. Due to their somatic illness resistance and low respiratory demands, they can withstand harsh environments, including low oxygen and high ammonia levels2. Diseases caused by parasites in fish are common worldwide, particularly in the tropics3. One of the most common and effective ways that living things survive is through parasitism4. Therefore, fish parasitology is a crucial tool in research on aquatic health for developing a control strategy that requires a solid grasp of parasite biology5,6. The health of people and domesticated animals as well as the safety of food, and the aquaculture world are all threatened by fish-borne trematodes, especially those that are zoonotic in origin (fish-borne zoonotic trematodes, or “FZT”)7,8,9.
Second intermediate hosts can include a wide variety of creatures, such as snails, bivalves, aquatic insect larvae, crustaceans, frogs, fish, and reptiles which infect the fish with encysted metacercariae. More importantly, many fish parasites, especially trematodes, are major zoonotic agents that can seriously harm human health by causing diarrhea and abdominal pain. The major way that humans become infected is through eating raw or inadequately cooked fish that contains encysted metacercariae. Recent additions to the list of emerging infectious illnesses include fish-borne zoonotic trematodes, according to the World Health Organization and the United Nations Food Agriculture Organization10.
Cytokines are released by immune cells that have been triggered in response to a variety of pathogens, such as parasitic, bacterial, or viral components11. When they attach to the appropriate receptors, they can modify immune responses in an autocrine or paracrine manner. Interferons (IFNs), interleukins (ILs), and tumor necrosis factors (TNFs) are all considered stimulating factors, while chemokines are different types of cytokines that are produced by macrophages, lymphocytes, granulocytes, mast cells, and epithelial cells12,13,14. IL-1, IL-6, and TNF-α are all necessary for pathogen elimination and the recruitment of macrophages, neutrophils, and lymphocytes to the diseased tissues in innate immunity15.
The major histocompatibility complex (MHC) genes play a critical role in adaptive immunity. Class I and class II genes, which make up the two main antigen-presenting groups of MHC molecules, have different functions, structures, and patterns of expression. The primary role of MHC class II genes is to encode cell-surface glycoproteins that bind foreign peptides to present self- and non-self peptides to the T-cell receptor of CD-4, which in turn triggers a particular immune response against the pathogen from which the peptides are derived. Previous research has demonstrated that the immune system’s cells, including antigen-presenting cells, are primarily found in the kidney, gut, gills, and spleen, where MHC genes are primarily expressed15.
One of the most important elements of the fish’s non-specific immune system is lysozyme, a polypeptide with a molecular weight of 14–18 kDa. Many different vertebrates, including freshwater and marine fish, contain the enzymes. Following the destruction of the outer wall by complement and other enzymes, it acts directly on different pathogens such as Gram-positive and negative bacteria. White blood cells, particularly neutrophils, and monocytes, as well as leukocyte-rich organs such as the kidney, spleen, skin mucus, gills, and digestive system, release lysozyme, which has antibacterial properties15.
Oreochromis niloticus experiences distinct reactions in response to different stressors in case of infection, which increase the levels of plasma cortisol, the primary hormone that activates glucose, and consequently glucose levels. Fish respond to stress by elevating blood levels of glucose and cortisol. Chronic stressors cause the release of cortisol. Stress is indicated by elevated plasma cortisol and glucose levels16.
Therefore, this work aims to identify the relationship between the different cell-mediated immune responses of Nile Tilapia (O. niloticus) infected with encysted metacercariae, liver enzymes analyses, lysozyme activity as well as blood glucose and cortisol levels of the infected fish as a reaction from the body against parasitic infection.
Materials and methods
Collection of fish
One hundred and twenty Nile Tilapia (O. niloticus) were sampled from various farms located in Kafr El-Sheikh Governorate (31.3° N 30.93° E) in Egypt between January 2021 and January 2022. The examined fish ranged in total length from 8 to 16 cm and weight from 50 to 85 g.
These fish were transferred alive for examination of encysted metacercariae (EMC) in different organs. The fish were taken to the lab while still alive to be examined for any parasites and to study the response of the immune system17. Fish were brought to the lab alive in aquariums filled with recirculating water and an oxygen source.
Parasitological examination
Each fish was euthanized using an overdose of commercial clove oil (Ectyo-colve®, France using at a concentration of 1239 ppm and a ratio of 1:4 to anhydrous ethanol); then the fish was necropsied and the mucous surrounding the skin, gills; fins, muscles around the head, muscles around the abdomen, liver, kidney, spleen, intestine, and eyes were examined under a light microscope (Olympus CX41 microscope; Japan). The muscles were compressed between two slides and examined under a stereoscopic microscope for the presence of any encysted metacercaria (EMC)18,19,20.
Quantitive real-time polymerase chain reaction (qRT-PCR)
Sampling
Under sanitary conditions, samples from the gills, spleen, liver, kidney, and muscles infected with encysted metacercariae (EMC) were collected. Five non-infected control fish were sampled in the same way.
RNA isolation
The sampled fish (five infected fish) selected for expression analysis harbored only encysted metacercariae (EMC). The mRNA from 100 mg of the examined samples was isolated using a total RNA isolation kit (Ambion, Applied Biosystems), following the manufacturer’s instructions. The tissues were homogenized in a FastPrep-24 homogenizer using Lysing Matrix D tubes (MP Biomedicals)21. Using Nanodrop, the generated mRNA purity and quantity were evaluated (Thermo Scientific). Using the instructions provided in the manufacturer’s procedure, the High-Capacity cDNA Archive Kit (Applied Biosystems) reverse-transcribed the DNaseI-treated mRNA22.
The protocol of the q-Rt-PCR
According to the sequences particularly for O. niloticus that were deposited in the GenBank and listed in Table 123,24, PCR primers for various studied genes were created. Following the procedures of Akbari et al.25, for cDNA synthesis 2 μL of RNA extract was added to a hexamer primer solution to create cDNA; the mixture was then immediately put on ice for at least one minute after being incubated at 65 °C for five minutes in a thermal cycler. Ten microliters of a first standard reaction (2×) containing reverse transcriptase (2 μL), 10 mM MgCl2, and 1 mM dNTPs were incubated at 25 °C for 10 min, the PCR programming cycle were followed according to Attia et al.26.
Determination of liver enzymes, glucose, and cortisol levels
Blood samples from five infected and five non-infected fish were subjected to biochemical analysis. Fish given benzocaine (50 mg/L) for anesthesia were used for blood collection from caudal veins and blood was drawn with and without an anticoagulant. To obtain serum, blood was allowed to coagulate at 4 °C, centrifuged for 15 min at 1500 rpm, and then maintained frozen at − 20 °C for biochemical examination. Using commercial kits (Spectrum-diagnostics, Egypt) and a JASCO V-730 spectrophotometer (JASCO, Tokyo, Japan), glucose, Alanine aminotransferase (ALT), and aspartate aminotransferase (AST) levels were calculated. A commercially available cortisol kit was used to test blood cortisol levels by radioimmunoassay, and a liquid scintillation counter was used to measure radioactivity27.
Assessment of serum lysozyme activity
Based on the lysis of Micrococcus lysodeikticus (Sigma, USA), serum lysozyme activity was determined following Dotta et al.28, with some changes. 0.2 mg/mL PBS, pH 6.2, and 0.75 mL of M. lysodeikticus solution were combined with 0.25 mL of serum. The reaction was conducted at room temperature, and from 0 to 20 min, the absorbance at 450 nm was determined (Photometer, BM Co. Germany). Lyophilized chicken egg-white lysozyme was used to create a calibration curve that was used to determine the serum lysozyme concentrations (Sigma, USA)29.
Statistical analysis
All data were presented as range (mean ± standard error) using a Independent Sample T-test which was performed to compare the presence of immunological genes in different tissues between infected and non-infected groups.
Ethics approval, and consent to participate
This study was approved by the Institutional Animal Care and Use Committee, Faculty of Medicine, Assiut University, Egypt, (IRB No. 17300859).
Compliance with relevant guidelines and regulations
Clinical examination, dissection, sampling, sample processing, microscopical examination, physiological and immunological analyses were carried out in accordance with relevant guidelines and regulations supported by relevant references throughout the manuscript materials and methods section.
Compliance with ARRIVE guidelines
The current study was carried out in compliance with the ARRIVE guidelines when relevant methods were applied.
Results
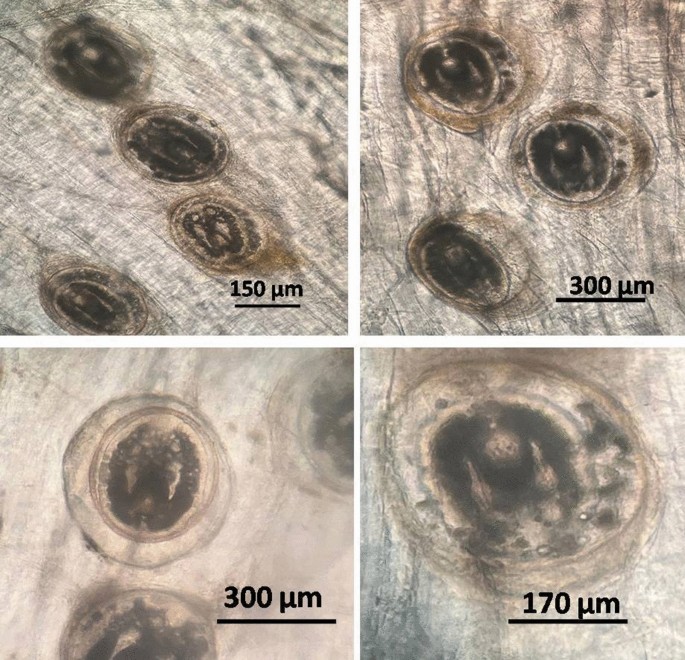
The collected encysted metacercariae were of the family Cyathocotylidae (Prohemistomum vivax). Prohemistomum vivax encysted metacercariae were discovered in different fish organs (muscles, skin, eyes, intestine, liver, kidney, and gills) of infected O. niloticus, which is oval to rounded in its shape, the P. vivax measured 335–385 (378 ± 5.6) μm in length and 315–320 (310 ± 5.5) μm in breadth. The cysts had a firm inner wall and a brittle double wall on their outer layer, which was brownish. The cyst was surrounded by two lobulated sacs on either side (Fig. 1).
Prohemistomum vivax encysted metacercariae collected from infected muscles.
The encysted metacercariae of Prohemistomum vivax were recorded during one year with 25% (30/120 fish) of the investigated samples having intensity of infection with 1–10 cysts per microscopic field.
The transcriprit of MHC-IIα were upregulated in different fish organs than in the control non-infected fish, the muscles, kidney, and spleen were the highest upregulation than the liver and gills (Fig. 2). Dealing with TLR-7 gene was upregulated in different organs as the muscles, spleen and liver were higher in upregulation than the kidney and gills (Fig. 2). The CD4 gene was upregulated in the spleen followed by the liver and muscles which higher than the kidney and gills (Fig. 2). The IL-8 gene was upregulated in the spleen followed by the muscles and kidney which higher than the liver and gills (Fig. 2).

Transcription analyses of different organs with different genes.
The mean value of lysozyme level in the serum of examined O. niloticus infected with EMC (P. vivax) was 155–159 (156.66 ± 1.2) μg/mL, while in the control non-infected fish it was 85–87 (86 ± 0.6) μg/mL.
In comparison to the control non-infected fish, the infected fish had significantly higher levels of AST, ALT, cortisol, and glucose Table 2.
Discussion
In this study, several encysted metacercariae in different organs were recorded and isolated for identification of the species which found it belonged to the Cyathocotilade family (P. vivax). We found a significant relationship between different criteria as parasites, and immunogenic relationships all of which had retard effects on the condition of the tissues.
The mRNA expression levels of cytokines and inflammatory molecules, including Toll-like receptors (TLRs) and cell signaling molecules involved in innate fish immune responses have been extensively studied30. In teleosts, the head kidney serves as the major lymphoid tissue compared to the bone marrow in vertebrates31. Since TLRs constitute a significant subset of pattern recognition receptors (PRRs), activating them can cause macrophages to create inflammatory cytokines, which in turn trigger the synthesis of chemokines derived from macrophages and increase the phagocytic activity of macrophages32,33. Leukocyte mobility is aided by chemokines in response to pathogenic exposure and controls immunological responses in this way. Several fish species can develop adaptive immune responses in their systemic organs following sub-lethal infection34,35. Some soluble and membrane-bound proteins work with the complement system, a crucial component of the innate immune system, to destroy pathogens36,37. Microorganisms and antibody-antigen (Ag-Ig) complexes can both directly activate complement-mediated death38.
In this study, we evaluate several immunological genes in different infected organs: gills, muscles, spleen, liver, and kidney. Fish gills are frequently infected with parasites, yet these areas serve as barriers to other parasites by secreting mucus, which reduces the parasite load. In response to parasitic protozoan infection, cultured O. niloticus can respond immunologically and upregulate the expression of several genes39.
By secreting various immunoglobulins, lectins, lysozymes, and complement C-reactive protein, mucus serves as the first immunological barrier of the body to protect against various infections, according to Zhu et al.12 and Jones39. These results mean that in the skin, gills, muscles, and liver the upregulation was higher due to the presence of mucous and macrophages as reported by Zhu et al.12, which explains different cytokines which secrete several products from mast cells, macrophages, and lymphocytes. These products were interferons, interleukins, and tumor necrosis factors.
Due to their critical function in immune protection and illness management, MHC genes have garnered a great deal of research. One of the most crucial characteristics of MHC genes is high polymorphism, which serves as the foundation for searching for gene markers linked to disease resistance. Class A and B genes in the MHC- II family, which encode the a and b chains, are significant members of the MHC family.
They both combine to produce class II heterodimers, which result in a useful protein that is present on the cell surface. They are crucial in bringing extracellular pathogens’ foreign peptides together and presenting them to helper T cells.
Many pathological and ecotoxicological studies have used hematological indicators as biomarkers of fish health status. In the current study, two groups, i.e. non-infected fish and infected ones, were examined for certain physiological factors connected to the liver of Nile Tilapia, including alanine aminotransferase, which elevated in the infected fish than the control non-infected ones as a result of an infection in the kidney and liver with malfunction. Other investigations have shown that these enzymes are elevated in Nile Tilapia, Oreochromis niloticus, in response to parasite pollutions like external protozoa and monogenetic trematodes40, which are in agreement with our findings. This can be brought on by the liver producing more enzymes or by hepatic cells being damaged41. An essential enzyme in the metabolism of amino acids is aspartate aminotransferase, which catalyzes the reversible transfer of an a-amino group between aspartate and glutamate.
The innate immune system’s key defense molecule, lysozyme, is crucial for mediating protection against microbial invasion. It is a leucocytic-derived mucolytic enzyme, so the lysozyme is greatly elevated than normal against severe infection with EMC42,43.
Conclusion
From the previous explanation of the infection of O. niloticus with the high prevalence of the parasitic disease; these parasites may affect human health. This destruction is present in the fish tissues and appeared in lysozyme activity and kidney and liver function. So, the water in the aquarium must be changed and cleaned regularly, and the wildlife and snails in the fish ponds must be cleaned regularly. Different rules in sanitation and health condition for ponds must be taken into consideration.
Data availability
All data generated or analyzed during this study are included in this article.
References
-
Smith, G., Smith, I. & Armstrong, S. The relationship between river flow and entry to the Aberdeenshire Dee by returning adult Atlantic salmon. J. Fish Biol. 45, 953–960 (1994).
Google Scholar
-
Zhu, C., Wang, Y. & Yu, N. Patterns in High Production and Ecological Fisheries in Lake Gehu, Jiangsu (Chinese Agriculture Press, 1997).
-
Roberts, L. S., Schmidt, G. & Janovy, J. Foundations of Parasitology 6th edn. (McGraw Hill, 2000).
-
Kabata, Z. Parasites and Diseases of Fish Cultured in the Tropics 318 (Taylor & Francis, 1985).
-
Bush, A. O., Fernandez, J. C., Esch, G. W., Seed, J. R. & Ndez, J. C. F. Parasitism: The Diversity and Ecology of Animal Parasites (Cambridge University Press, 2001).
-
Moraes, F. & Martins, M. Predisposing conditions and principal diseases of intensive fish farming teleosts. In Cyrino, JEP; Urbinatti, EC; Fracalossi, DM 343–383 (2004).
-
Anh, N. T. L. et al. Animal reservoir hosts and fish-borne zoonotic trematode infections on fish farms, Vietnam. Emerg. Infect. Dis. 15, 540–548 (2009).
Google Scholar
-
Youssef, A. I. & Uga, S. Review of parasitic zoonoses in Egypt. Trop. Med. Health 42, 3–14 (2014).
Google Scholar
-
Elsheikha, H. M. & Elshazly, A. M. Preliminary observations on infection of brackish and fresh water fish by heterophyid encysted metacercariae in Egypt. Parasitol. Res. 103, 971–977 (2008).
Google Scholar
-
Abd-ELrahman, S. M. et al. Prevalence and morphological investigation of parasitic infection in freshwater fish (Nile tilapia) from Upper Egypt. Animals 13, 1088. https://doi.org/10.3390/ani13061088 (2023).
Google Scholar
-
Salazar-Mather, T. & Hokeness, K. Cytokine and chemokine networks: Pathways to antiviral defense. In Chemokines and Viral Infection (ed. Lane, T. E.) 29–46 (Springer, 2006).
Google Scholar
-
Zhu, L.-Y., Nie, L., Zhu, G., Xiang, L.-X. & Shao, J.-Z. Advances in research of fish immune-relevant genes: A comparative overview of innate and adaptive immunity in teleosts. Dev. Comp. Immunol. 39, 39–62 (2013).
Google Scholar
-
Li, M.-F. & Zhang, J. CsTNF1, a teleost tumor necrosis factor that promotes antibacterial and antiviral immune defense in a manner that depends on the conserved receptor binding site. Dev. Comp. Immunol. 55, 65–75 (2016).
Google Scholar
-
Velázquez, J. et al. Novel IFNγ homologue identified in Nile tilapia (Oreochromis niloticus) links with immune response in gills under different stimuli. Fish Shellfish Immunol. 71, 275–285 (2017).
Google Scholar
-
Zou, J. & Secombes, C. J. The function of fish cytokines. Biology 5, 23. https://doi.org/10.3390/biology5020023 (2016).
Google Scholar
-
Barreto, R. E. & Volpato, G. L. Stress responses of the fish Nile tilapia subjected to electroshock and social stressors. Braz. J. Med. Biol. Res. 39, 1605–1612. https://doi.org/10.1590/s0100-879×2006001200012 (2006).
Google Scholar
-
Attia, M. M., Elgendy, M. Y., Prince, A., El-Adawy, M. M. & Abdelsalam, M. Morphomolecular identification of two trichodinid coinfections (Ciliophora: Trichodinidae) and their immunological impacts on farmed Nile Tilapia. Aquac. Res. 52, 4425–4433 (2021).
Google Scholar
-
Yamaguti, S. Systema Helminthum. Vol. I. The Digenetic Trematodes of Vertebrates-Part II (1958).
-
El-Seify, M. A., Sultan, K., Elhawary, N. M., Satour, N. S. & Marey, N. M. Prevalence of heterophyid infection in tilapia fish “Orechromas niloticus” with emphasize of cats role as neglected reservoir for zoonotic Heterophyes heterophyes in Egypt. J. Parasit. Dis. 45, 35–42 (2021).
Google Scholar
-
Paperna, I. Parasite, infections and disease of fishes in Africa—An update. CIFA Tech. Pap. 31, 1–220 (1996).
-
Younis, N. A., Laban, S. E., Al-Mokaddem, A. K. & Attia, M. M. Immunological status and histopathological appraisal of farmed Oreochromis niloticus exposed to parasitic infections and heavy metal toxicity. Aquacult. Int. 28, 2247–2262 (2020).
Google Scholar
-
Picard-Sánchez, A. et al. Acquired protective immune response in a fish-myxozoan model encompasses specific antibodies and inflammation resolution. Fish Shellfish Immunol. 90, 349–362 (2019).
Google Scholar
-
Praveen, K., Evans, D. L. & Jaso-Friedmann, L. Constitutive expression of tumor necrosis factor-alpha in cytotoxic cells of teleosts and its role in regulation of cell-mediated cytotoxicity. Mol. Immunol. 43, 279–291 (2006).
Google Scholar
-
Heinecke, R. D. & Buchmann, K. Inflammatory response of rainbow trout Oncorhynchus mykiss (Walbaum, 1792) larvae against Ichthyophthirius multifiliis. Fish Shellfish Immunol. 34, 521–528 (2013).
Google Scholar
-
Akbari, M., Taghizadeh, V., Heidarieh, M. & Hajimoradloo, A. The key role of tumor necrosis factor alpha (TNF-α) in vaccinated rainbow trout via irradiated Ichthyophthirius multifiliis trophont. Vet. Arch. 87, 229–237 (2017).
Google Scholar
-
Attia, M. M., Abdelsalam, M., Elgendy, M. Y. & Sherif, A. H. Dactylogyrus extensus and Pseudomonas fluorescens dual infection in farmed common carp (Cyprinus carpio). Microbial Pathog. 173, 105867. https://doi.org/10.1016/j.micpath.2022.105867 (2022).
Google Scholar
-
Liu, Y. et al. Impaired peroxisomal fat oxidation induces hepatic lipid accumulation and oxidative damage in Nile tilapia. Fish Physiol. Biochem. 46, 1229–1242 (2020).
Google Scholar
-
Dotta, G. et al. Leukocyte phagocytosis and lysozyme activity in Nile tilapia fed supplemented diet with natural extracts of propolis and Aloe barbadensis. Fish Shellfish Immunol. 39, 280–284 (2014).
Google Scholar
-
Saurabh, S. & Sahoo, P. Lysozyme: An important defence molecule of fish innate immune system. Aquac. Res. 39, 223–239 (2008).
Google Scholar
-
Dickerson, H. & Findly, R. Immunity to Ichthyophthirius infections in fish: A synopsis. Dev. Comp. Immunol. 43, 290–299 (2014).
Google Scholar
-
Hansen, J. D. & Zapata, A. G. Lymphocyte development in fish and amphibians. Immunol. Rev. 166, 199–220 (1998).
Google Scholar
-
Alvarez-Pellitero, P. Fish immunity and parasite infections: From innate immunity to immunoprophylactic prospects. Vet. Immunol. Immunopathol. 126, 171–198 (2008).
Google Scholar
-
Jun, W. et al. Molecular characterization of an IL-1β gene from the large yellow croaker (Larimichthys crocea) and its effect on fish defense against Vibrio alginolyticus infection. Zool. Res. 36, 133 (2015).
Google Scholar
-
Dickerson, H. W. The biology of teleost mucosal immunity. In Fish Defenses: Pathogens, Parasites and Predators 1–42 (2009).
-
Jørgensen, L. V. G., Korbut, R., Jeberg, S., Kania, P. W. & Buchmann, K. Association between adaptive immunity and neutrophil dynamics in zebrafish (Danio rerio) infected by a parasitic ciliate. PLoS ONE 13, e0203297 (2018).
Google Scholar
-
Gasque, P. Complement: A unique innate immune sensor for danger signals. Mol. Immunol. 41, 1089–1098 (2004).
Google Scholar
-
Morgan, B. P., Marchbank, K. J., Longhi, M. P., Harris, C. L. & Gallimore, A. M. Complement: Central to innate immunity and bridging to adaptive responses. Immunol. Lett. 97, 171–179 (2005).
Google Scholar
-
Holland, M. C. H. & Lambris, J. D. The complement system in teleosts. Fish Shellfish Immunol. 12, 399–420 (2002).
Google Scholar
-
Jones, S. R. The occurrence and mechanisms of innate immunity against parasites in fish. Dev. Comp. Immunol. 25, 841–852 (2001).
Google Scholar
-
Younis, A. Effect of some ectoparasites on the blood and serum constituents of Oreochromis niloticus fish with referring to treatment. Beni Suif Vet. Med. J. 9, 341–351 (1999).
-
Yang, J.-L. & Chen, H.-C. Serum metabolic enzyme activities and hepatocyte ultrastructure of common carp after gallium exposure. Zool. Stud. 42, 455–461 (2003).
Google Scholar
-
Jollès, P. & Jollès, J. What’s new in lysozyme research? Mol. Cell. Biochem. 63, 165–189 (1984).
Google Scholar
-
Nelson, D. L. & Cox, M. M. Lehninger Principles of Biochemistry 623–658 (Worth Publishers, 2000).
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
This study was conducted in cooperation with all authors. All authors were involved in the research design and concept. Dr. N.A.Y. conducted fish sampling. Dr. H.T. and M.M.A. conducted the identification of the parasites using Light microscopy. M.M.A. conducted immunological studies. Dr. S.I.E.-S. conducted physiological studies. All authors participated in the writing of the manuscript as well as revising it.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
Reprints and Permissions
About this article
Cite this article
Younis, N.A., Thabit, H., El-Samannoudy, S.I. et al. The immune responses of Oreochromis niloticus against Prohemistomum vivax encysted metacercariae infection with the evaluation of different biomarkers stressors.
Sci Rep 13, 11885 (2023). https://doi.org/10.1038/s41598-023-38809-z
-
Received: 27 September 2022
-
Accepted: 14 July 2023
-
Published: 23 July 2023
-
DOI: https://doi.org/10.1038/s41598-023-38809-z
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.