Cancer and neoplasms
Clinical outcome of Mantle Cell Lymphoma patients with high-risk disease (high-risk MIPI-c or high p53 expression)
Abstract
Currently, treatment allocation of patients with Mantle Cell Lymphoma (MCL) is mainly based on age and medical fitness. The combined MCL International Prognostic Index (MIPI-c) allows to predict prognosis using clinical factors (MIPI) and the Ki-67 index. However, high p53 expression as surrogate for TP53 alterations has demonstrated to be an independent predictor for poor outcome. We aimed to define a clear high-risk group based on the combination of MIPI, Ki-67 and p53 expression/TP53 alteration. A total of 684 patients from the prospective European MCL-Younger and MCL-Elderly trials were evaluable. The classification of high-risk disease (HRD) as high-risk MIPI-c or p53 expression >50% versus low-risk disease (LRD) as low, low-intermediate or high-intermediate MIPI-c and p53 expression ≤50% allowed to characterize two distinct groups with highly divergent outcome. Patients with HRD had significantly shorter median failure-free survival (FFS) (1.1 vs. 5.6 years, p < 0.0001) and overall survival (OS) (2.2 vs. 13.2 years, p < 0.0001) compared to those with LRD. These major differences were confirmed in two validation cohorts from the Italian MCL0208 and the Nordic-MCL4 trials. The results suggest that this subset of HRD patients is not sufficiently managed with the current standard treatment and is asking for novel treatment strategies.
Introduction
Mantle cell lymphoma (MCL) is a rare and commonly aggressive subtype of B-cell lymphoma characterized by the translocation t(11;14) with consecutive cyclin D1 overexpression. The clinical course is heterogeneous and marked by recurring relapses. Novel therapeutic strategies such as the addition of high-dose cytarabine to the induction treatment prior to autologous stem cell transplantation (aSCT), Rituximab (R) maintenance and the approval of Bruton’s tyrosine kinase inhibitors (BTKi) substantially improved the survival of patients [1,2,3]. Currently, patients are allocated for treatment mainly considering age, stage and performance status.
The Mantle Cell Lymphoma International Prognostic Index (MIPI) allows for discriminating prognostic risk groups based on age, performance status, lactate dehydrogenase (LDH) and leukocyte count [4], the combined MIPI-c additionally incorporates the Ki-67 index [5]. In prospective trials, the biological risk factors Ki-67, blastoid or pleomorphic cytology [5], and TP53 alterations [6,7,8] were associated with inferior outcome independent of MIPI. The prognostic relevance of MCL cytology is closely correlated to the Ki-67 index, which is generally increased in blastoid MCL [5, 9, 10]. P53 expression is a widely applicable diagnostic method serving as surrogate marker for TP53 alterations [11, 12]. In univariate analyses TP53 alterations, such as mutations and deletions, were both validated as negative predictor for outcome in the Nordic MCL2, MCL3, and MCL4 as well as in the European MCL Younger and Elderly trial cohort [7, 8, 13]. Thus, even more intensive regimens including high-dose cytarabine and aPBSCT fail to overcome the dismal prognosis of TP53 alterations [7, 8].
New concepts are urgently needed to define a more refined high-risk population and identify effective treatment strategies for these patients. In this study, we aimed to define a combination of MIPI, Ki-67 and p53 expression/TP53 alterations that reliably identifies a high-risk group.
Methods
Patients
A total of 1183 MCL patients with confirmed MCL and Ann Arbor stage II to IV were registered in the MCL Younger [14] (NCT00209222) and MCL-Elderly trial [15] (NCT00209209) of the European MCL Network from 2004–2010 serving as training cohort. Patients without available Ki-67 and p53 data were excluded from this analysis. All patients gave written informed consent to participation in the trials.
Two independent series of 300 patients from the MCL0208 trial [16] and 51 patients from the Nordic-MCL4 trial [17] were included in this study as validation cohort. The MCL0208 cohort considered TP53 mutation or del(17p) and the Nordic-MCL4 cohort only TP53 mutation status instead of p53 expression data.
Immunohistochemistry
Immunohistochemistry was performed centrally on either tissue microarrays or whole tissue sections. P53 was stained with a mouse monoclonal antibody (Leica/NovoCastra clone DO7) and scored as negative (0%), low (1–10%), intermediate (10–50%) or high (>50%) by one observer based on visual assessment as described [6]. Ki-67 index was centrally assessed in accordance with established guidelines [18].
Pathology
Formalin-fixed paraffin-embedded (FFPE) diagnostic biopsy material was classified as classical or pleomorphic/blastoid variant by cytomorphological features confirmed by the central pathology review of a pathology reference center (European MCL Pathology Panel).
Risk variables
Biological risk variables Ki-67 and p53 expression along with the clinical prognostic tool MIPI were investigated in various combinations. MIPI score is the weighted sum of MIPI prognostic factors weighted by the regression coefficients from the defining Cox regression model [4]. The equally weighted combination of MIPI with the dichotomized Ki-67 index at the validated 30% cutoff defines the MIPI-c [5].
It was previously reported that none of the patients in the MCL Younger and Elderly cohort with complete absence of p53 expression did display TP53 deletions [6, 8]. For this reason, we focused on the cutoff for p53 expression at 50% serving as biomarker indicating a TP53 mutation with altered functions [11, 19].
Combinations tested to define high-risk disease (HRD) were p53 expression >50% or Ki-67 ≥ 30% (definition 1), p53 expression >50% or high, high-intermediate MIPI-c (definition 2), or p53 expression >50% or high MIPI-c (definition 3). Accordingly, low-risk disease (LRD) was defined by p53 expression ≤50% and Ki-67 < 30% (definition 1), p53 expression ≤50% and low, low-intermediate MIPI-c (definition 2) or p53 expression ≤50% and low, low-intermediate, high-intermediate MIPI-c (definition 3).
For the validation cohort, presence of TP53 mutation and del(17p) (only MCL0208 cohort) defined HRD instead of high p53 expression.
Statistical methods
Cases with missing data for both Ki-67 and p53 expression or TP53 mutation/deletion, respectively, were excluded from the analysis. If one high-risk feature applies, missing data for the other variable is allowed. For the classification of LRD all variables must be available. The percentages of HRD in the study population were estimated based on complete cases, where patients with missing data in either Ki-67 or p53 expression were excluded in order to minimize bias. The number of complete cases is significantly smaller but is more reliable to estimate the true proportion of MCL patients with HRD. Analyses for the outcomes were performed on all the classifiable patients with available outcome data. In addition, we performed sensitivity analyses with complete cases.
We estimated and compared failure-free survival (FFS, defined as time from treatment start to stable disease, progression, or death from any cause, whichever occurred first) and overall survival (OS, defined as time from study registration to death from any cause) stratified by Ki-67 index (50%), the combination of Ki-67 and p53 and MIPI-c and p53 using Kaplan-Meier-plots, logrank tests, and Cox regression. Five-year FFS and OS probabilities were reported along with median FFS and OS times. Quantification of follow-up was done by the reversed Kaplan-Meier method. Status of the clinical data was that of July 08, 2021, the latest available time point of medically reviewed data from MCL Younger and MCL-Elderly trials.
Results
Six hundred eighty-four patients (MCL Younger n = 390, MCL Elderly n = 294) out of 1183 registered study patients with MCL from the training cohort had evaluable data either for Ki-67 or p53 (Supplemental Fig. 1). Among these, low-risk MIPI was more frequent (43% vs. 27%), whereas high-risk MIPI was significantly less frequent (24% vs. 44%, p < 0.0001) compared to those without evaluable data (Table 1). Accordingly, median FFS and OS was superior in the subgroup of patients with available data (4.4 vs. 3.2 years, p = 0.0066 and 9.6 vs. 6.6 years, p = 0.0013) (Supplemental Fig. 2). This difference is mainly explained by an overrepresentation of patients with available pathology data from the MCL Younger trial (57% vs 45%) who had a better overall outcome. Of note, there was no difference in outcome of patients with a high MIPI comparing those with available data for Ki-67/p53 vs. unavailable data for Ki-67 and p53.
Apart from MIPI parameters, patient characteristics were equally distributed among the two groups (Table 1). High MIPI-c was seen in 63 of 612 cases (10%) and high p53 expression in 54 of 348 (16%) cases. High p53 expression was associated with inferior median FFS (1.5 vs. 4.6 years; p < 0.0001) and OS (2.8 vs. 10.7 years, p < 0.0001) compared to p53 expression ≤50% (data not shown). Blastoid cytology was no negative predictor in patients with low Ki-67 (Supplementary Fig. 3).
Using Ki-67 ≥ 30% or high p53 expression to define HRD (definition 1) resulted in a relatively large high-risk group with 37% of complete cases. Median FFS and OS was 1.8 vs. 6.0 years (HR 2.01, p < 0.0001) and 4.0 vs. 14.4 years (HR 2.57, p < 0.0001) compared to LRD (Supplemental Fig. 4). Considering also clinical factors, we tested the impact of high, high-intermediate MIPI-c or high p53 expression (definition 2) on outcome. This high-risk definition includes 41% of complete cases and revealed similar results (median FFS: 1.7 vs. 6.0 years, HR 2.40, p < 0.0001; median OS: 3.6 vs. 15.4 years, HR 3.24, p < 0.0001) (Supplemental Fig. 5).
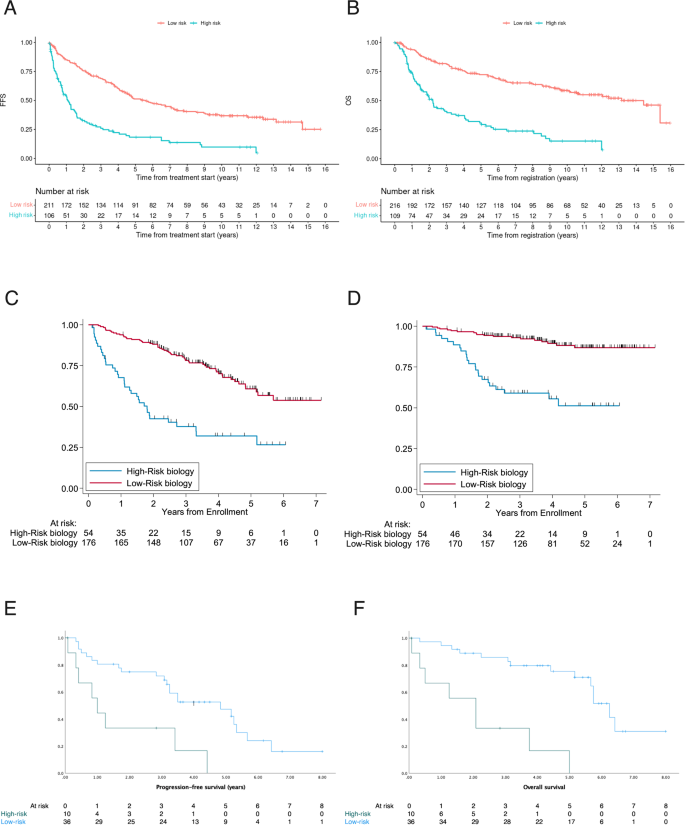
The combination of high MIPI-c or high p53 expression (definition 3) defined the smallest group of high-risk patients and had the highest discriminatory power between HRD and LRD in terms of the hazard ratios for FFS and OS, why we chose that definition for further analyses. Based on the selection process of definition 3, 22% complete cases could be assigned to the HRD group (n = 60) and 78% (n = 216) had confirmed LRD. After a median follow-up of 9.6 (FFS) and 9.4 (OS) years the median FFS (1.1 years vs. 5.6 years; HR 2.97, p < 0.0001; Fig. 1A) and OS (2.2 vs. 13.2 years, HR 3.69, p < 0.0001; Fig. 1B) was significantly decreased in the high-risk compared with the low-risk group. 5-year and 10-year FFS probabilities were 18% vs. 51% and 10% vs. 37%; 5-year and 10-year OS probabilities were 31% vs. 72% and 15% vs. 59% for HRD vs. LRD, respectively. Sensitivity analyses in complete cases showed similar results to the analyses in classifiable cases. These aforementioned significant differences were observed consistently across both trial groups, regardless of whether patients received conventionally dosed immunochemo- and maintenance therapy in MCL Elderly (median FFS: 0.8 vs. 3.9 years, p < 0.0001; median OS: 1.9 vs 9.7 years, p < 0.0001) or received induction with intention to high-dose chemotherapy followed by aSCT in MCL Younger (median FFS: 1.9 vs. 6.7 years, p < 0.0001; median OS: 3.0 years vs. median not reached, p < 0.0001) (Fig. 2).
Prognostic impact of high MIPI-c or high p53 expression/TP53 mutation (definition 3) in the training (A, B) and the validation cohorts (C–F). Kaplan–Meier estimates of FFS (A) and OS (B) of patients with high MIPI-c or p53 expression >50% (high-risk disease) compared to low, low-intermediate or high-intermediate MIPI-c and p53 expression ≤50% (low-risk disease) from the MCL Younger and Elderly cohort. The number at risk is based on all classifiable patients. Estimates of PFS and OS in the MCL0208 (C, D) and the MCL4 (E, F) validation cohorts of patients with high MIPI-c or TP53 mutation and del(17p) (only MCL0208 cohort) (high-risk disease) compared to patients with low, low-intermediate or high-intermediate MIPI-c and no TP53 mutation and del(17p) (low-risk disease).

Kaplan–Meier estimates of FFS (A, C) and OS (B, D) among patients treated in the MCL Younger (A, B) and MCL Elderly (C, D) trial stratified by the presence of high MIPI-c or p53 expression >50% (high-risk disease) compared to low, low-intermediate or high-intermediate MIPI-c and p53 expression ≤50% (low-risk disease). The number at risk is based on all classifiable patients.
Subgroup analyses showed that R-CHOP compared to R-FC in MCL Elderly (median OS 1.1 vs. 2.3 years; HR 3.61 vs. 3.50) as well as R-CHOP/R-DHAP induction compared to R-CHOP induction in MCL Younger (median OS 1.5 vs. 5.6 years; HR 5. 94 vs. 3.06.) could partially mitigate the dismal prognosis of HRD. However, the superior treatment arms failed to fully compensate for the poor prognostic impact of HRD (Fig. 3).

Kaplan–Meier estimates of OS among patients treated with R-CHOP induction and aPBSCT (A), alternating R-CHOP/R-DHAP induction and aPBSCT (B), R-FC and IFN maintenance (C) and R-CHOP + R maintenance (D) stratified by the presence of high MIPI-c or p53 expression >50% (high-risk disease) compared to low, low-intermediate or high-intermediate MIPI-c and p53 expression <50% (low-risk disease). The number at risk is based on all classifiable patients.
The results were validated in independent series of 230 classifiable patients from the FIL-MCL0208 trial and 44 classifiable patients from the Nordic Lymphoma Group MCL4 trial. The MCL0208 trial enrolled patients aged 18–65 years undergoing cytarabine-containing induction and aSCT before randomization to 24 months of lenalidomide maintenance compared to observation [16]. The Nordic-MCL4 trial was carried out in patients >65 years not suitable for aSCT receiving the combination of lenalidomide and R-bendamustine [17]. HRD (definition 3) was present in 20% (MCL0208) and 23% (MCL4) of complete cases. Main patient features are shown in Supplemental Table 1 (MCL0208) and 2 (MCL4). Presence of HRD according to definition 3 conditioned significant inferior outcome in both, the MCL0208 cohort and the MCL4 cohort. Median Progression-free survival (PFS) was 1.8 years vs. 5.2 years (HR 3.52, p < 0.001; Fig. 1C) and 1.0 years vs. 4.8 years (HR 3.6, p = 0.002; Fig. 1E) for HRD vs. LRD in the MCL0208 and the MCL4 validation cohort. The adverse prognostic value also translated into OS with a hazard ratio for death of 5.5 (95% CI: 2.96–10.22, p < 0.001) (Fig. 1D) and 7.6 (95% CI: 2.8–20.8, p < 0.0001) (Fig. 1F) for HRD disease in the MCL0208 and the MCL4 series.
Discussion
To the best of our knowledge, this is the first investigation exploring a novel combination of the biological and clinical risk factors Ki-67, p53 expression/TP53 alterations and MIPI that is valid for young and elderly MCL patients. All patients were treated in prospective trials from the European Mantle Cell Lymphoma Network. The frequency of high MIPI-c and high p53 expression at 10% and 16% of the training cohort matches well with the Nordic MCL2 and MCL3 study cohort where the reported frequency of high MIPI-c and TP53 mutations was 13% and 16%, respectively [7].
The p53 tumor suppressor gene (TP53) is a crucial regulator of the cell cycle, apoptosis, DNA repair and senescence [20]. Genetic aberrations of TP53 such as point mutations and allelic deletions regularly emerge during tumorigenesis and result in a loss-of-function of the TP53 gene [21]. Consequently, the p53 protein often accumulates which can be visualized using p53-specific antibodies [11]. DNA sequencing is considered the most reliable method to analyze the TP53 mutation status. Immunohistochemistry has good correlation to TP53 missense mutations, but still misses up 18% of these mutations [22]. On the other hand, immunohistochemistry misses truncating mutations (non-missense) that lead to the lack of expression of the protein. These mutations may be up to 10–25% of the TP53 mutations in MCL and may represent up to 11% of patients with low protein expression [22, 23]. Of note, some patients with high p53 expression were reported to have wild type TP53. Considering these limitations, in the era of precision medicine, we recommend the molecular study of TP53 as already performed in clinical practice in chronic lymphocytic leukemia. However, p53 expression serving as surrogate for TP53 mutation status is characterized by the wide and practical availability. Accordingly, we recommend to assess both TP53 mutation status and p53 expression by IHC for all MCL patients. If either the molecular study indicates deletion/mutation or p53 expression is >50%, the disease should be considered high-risk.
While high p53 expression was an independent risk factor for poor outcome, blastoid cytology had no predictive value in patients with low Ki-67 < 30%. These results confirm that adding cytology, which is known to be poorly standardized, to the definition of HRD does not relevantly improve the results. However, it must be noted, that of 341 patients with Ki-67 < 30% and available morphology, only 16 had blastoid morphology. This leads to limited power to detect an effect of blastoid cytology. We recommend maintaining the determination of MCL morphology in clinical practice.
Considering the two biological risk factors high Ki-67 or p53 expression (definition 1) identified patients with a 2.5-fold higher risk of death compared to those without these risk factors. Adding also clinical risk factors according to definition 2 revealed a group of high-risk patients with a 3.2-fold higher risk of death. Definition 3 distinguished most clearly between high and low-risk patients with a 3-fold higher risk of treatment failure and a 3.7-fold higher risk of death for HRD. The frequency of high-risk disease according to definition 3 was consistent in the training as well as in the validation cohort. The dismal outcome of this high-risk group with half of the patients failing treatment after one and dying after 2 years, is in line with the data reported for patients with mutated TP53 in the MCL2 and MCL3 cohort [7]. The “MIPI-genetic” (“MIPI-g”) which was developed for younger MCL patients and adds KMT2D mutations and TP53 disruptions to MIPI-c identifies a high-risk group with a 4-year PFS and OS probability of 11.5% and 44.9% [12].
Also in the superior treatment arms of the MCL-Elderly and Younger Trial, the presence of HRD reliably predicted poor survival.
The vast majority of the patients in the MCL-Elderly and Younger Trial received conventional chemotherapy after the first relapse [24, 25]. While allogeneic or autologous stem cell transplantation was carried out in some patients of the Younger trial, only a minority received targeted therapies such as a BTKi (1–3% in the MCL Younger, 7–10% in the MCL-Elderly trial) [24, 25]. Hence, the efficacy of novel therapies in HRD patients can hardly be inferred from these trials as the numbers are too small.
Of note, also the immunomodulatory drug lenalidomide trial did not overcome the adverse impact of TP53 mutations in combination with R-bendamustine [13]. The general validity of the biology-based HRD model was confirmed in the FIL-MCL0208 and the Nordic-MCL4 trial with significant inferior PFS and higher risk for death in high-risk patients.
Interestingly, in relapsed or refractory MCL even the potent BTKi ibrutinib does not overcome the poor prognosis that is linked to TP53 mutations and high MIPI-c [26,27,28]. A benefit of ibrutinib for progression-free survival in the first line treatment of elderly patients was recently reported in the SHINE study (ClinicalTrials.gov Identifier: NCT01776840) [29]. However, the addition of ibrutinib to rituximab and bendamustine did not show a clear benefit in patients with TP53 mutations or a MIPI score indicating high-risk [29]. The benefit of ibrutinib in combination with chemotherapy in treatment-naive transplant eligible patients has been recently reported for the TRIANGLE study, but longer follow-up is required to evaluate whether this combination fully overcomes the biological risk factors [30]. Of note, neither Ki-67 ≥ 50%, TP53 mutation, nor intermediate or high MIPI had any negative prognostic value for 6-months PFS in relapsed or refractory patients treated with the novel CD19 directed CAR-T-cell therapy KTE-X19 [31], suggesting that cellular immunotherapy might overcome the poor prognosis in high-risk patients.
As MCL patients with HRD defined by high MIPI-c or high p53 expression/TP53 alteration had a dismal clinical course of the disease, we recommend to incorporate these factors in routine diagnostic practice as suggested by the WHO 5th edition and the International Consensus Classification (ICC) to identify patients with need for novel therapeutic strategies [32, 33]. At present, HRD MCL patients should be treated with ibrutinib-containing induction based on the SHINE and TRIANGLE data [29, 30]. However, we think that this approach will not completely overcome the dismal prognosis of high MIPI-c or high p53/TP53 mutation and clinical trials are needed that particularly address HRD patients.
In conclusion, the combination of the prognostic MIPI index with the biological risk factors TP53 mutation and high Ki-67 expression reliably defines a subset of MCL patients with dismal prognosis. On the other hand, patients without these high-risk features achieve an excellent outcome with an overall survival over a decade with the current standard of care. Furthermore, these results will allow risk stratification in clinical trials, to hopefully develop innovative therapies especially for the high-risk MCL population which has the greatest medical need.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
-
Castellino A, Wang Y, Larson MC, Maurer MJ, Link BK, Farooq U, et al. Evolving frontline immunochemotherapy for mantle cell lymphoma and the impact on survival outcomes. Blood Adv. 2022;6:1350–60.
-
Zhou K, Zou D, Zhou J, Hu J, Yang H, Zhang H, et al. Zanubrutinib monotherapy in relapsed/refractory mantle cell lymphoma: a pooled analysis of two clinical trials. J Hematol Oncol. 2021;14:167.
Google Scholar
-
Visco C, Di Rocco A, Evangelista A, Quaglia FM, Tisi MC, Morello L, et al. Outcomes in first relapsed-refractory younger patients with mantle cell lymphoma: results from the MANTLE-FIRST study. Leukemia. 2021;35:787–95.
Google Scholar
-
Hoster E, Dreyling M, Klapper W, Gisselbrecht C, van Hoof A, Kluin-Nelemans HC, et al. A new prognostic index (MIPI) for patients with advanced-stage mantle cell lymphoma. Blood. 2008;111:558–65.
Google Scholar
-
Hoster E, Rosenwald A, Berger F, Bernd HW, Hartmann S, Loddenkemper C, et al. Prognostic value of Ki-67 index, cytology, and growth pattern in mantle-cell lymphoma: results from randomized trials of the European Mantle Cell Lymphoma Network. J Clin Oncol. 2016;34:1386–94.
Google Scholar
-
Aukema SM, Hoster E, Rosenwald A, Canoni D, Delfau-Larue MH, Rymkiewicz G, et al. Expression of TP53 is associated with the outcome of MCL independent of MIPI and Ki-67 in trials of the European MCL Network. Blood. 2018;131:417–20.
Google Scholar
-
Eskelund CW, Dahl C, Hansen JW, Westman M, Kolstad A, Pedersen LB, et al. TP53 mutations identify younger mantle cell lymphoma patients who do not benefit from intensive chemoimmunotherapy. Blood. 2017;130:1903–10.
Google Scholar
-
Delfau-Larue MH, Klapper W, Berger F, Jardin F, Briere J, Salles G, et al. High-dose cytarabine does not overcome the adverse prognostic value of CDKN2A and TP53 deletions in mantle cell lymphoma. Blood. 2015;126:604–11.
Google Scholar
-
Raty R, Franssila K, Joensuu H, Teerenhovi L, Elonen E. Ki-67 expression level, histological subtype, and the International Prognostic Index as outcome predictors in mantle cell lymphoma. Eur J Haematol. 2002;69:11–20.
Google Scholar
-
Geisler CH, Kolstad A, Laurell A, Jerkeman M, Raty R, Andersen NS, et al. Nordic MCL2 trial update: six-year follow-up after intensive immunochemotherapy for untreated mantle cell lymphoma followed by BEAM or BEAC + autologous stem-cell support: still very long survival but late relapses do occur. Br J Haematol. 2012;158:355–62.
Google Scholar
-
Stefancikova L, Moulis M, Fabian P, Ravcukova B, Vasova I, Muzik J, et al. Loss of the p53 tumor suppressor activity is associated with negative prognosis of mantle cell lymphoma. Int J Oncol. 2010;36:699–706.
Google Scholar
-
Ferrero S, Rossi D, Rinaldi A, Bruscaggin A, Spina V, Eskelund CW, et al. KMT2D mutations and TP53 disruptions are poor prognostic biomarkers in mantle cell lymphoma receiving high-dose therapy: a FIL study. Haematologica. 2020;105:1604–12.
Google Scholar
-
Eskelund CW, Albertsson-Lindblad A, Kolstad A, Laurell A, Raty R, Pedersen LB, et al. Lenalidomide plus bendamustine-rituximab does not overcome the adverse impact of TP53 mutations in mantle cell lymphoma. Haematologica. 2018;103:e541–e3.
Google Scholar
-
Hermine O, Hoster E, Walewski J, Bosly A, Stilgenbauer S, Thieblemont C, et al. Addition of high-dose cytarabine to immunochemotherapy before autologous stem-cell transplantation in patients aged 65 years or younger with mantle cell lymphoma (MCL Younger): a randomised, open-label, phase 3 trial of the European Mantle Cell Lymphoma Network. Lancet. 2016;388:565–75.
Google Scholar
-
Kluin-Nelemans HC, Hoster E, Hermine O, Walewski J, Trneny M, Geisler CH, et al. Treatment of older patients with mantle-cell lymphoma. N Engl J Med. 2012;367:520–31.
Google Scholar
-
Ladetto M, Cortelazzo S, Ferrero S, Evangelista A, Mian M, Tavarozzi R, et al. Lenalidomide maintenance after autologous haematopoietic stem-cell transplantation in mantle cell lymphoma: results of a Fondazione Italiana Linfomi (FIL) multicentre, randomised, phase 3 trial. Lancet Haematol. 2021;8:e34–e44.
Google Scholar
-
Albertsson-Lindblad A, Kolstad A, Laurell A, Raty R, Gronbaek K, Sundberg J, et al. Lenalidomide-bendamustine-rituximab in patients older than 65 years with untreated mantle cell lymphoma. Blood. 2016;128:1814–20.
Google Scholar
-
Klapper W, Hoster E, Determann O, Oschlies I, van der Laak J, Berger F, et al. Ki-67 as a prognostic marker in mantle cell lymphoma-consensus guidelines of the pathology panel of the European MCL Network. J Hematop. 2009;2:103–11.
Google Scholar
-
Hernandez L, Fest T, Cazorla M, Teruya-Feldstein J, Bosch F, Peinado MA, et al. p53 gene mutations and protein overexpression are associated with aggressive variants of mantle cell lymphomas. Blood. 1996;87:3351–9.
Google Scholar
-
Vousden KH. Outcomes of p53 activation-spoilt for choice. J Cell Sci. 2006;119:5015–20.
Google Scholar
-
Hainaut P, Hollstein M. p53 and human cancer: the first ten thousand mutations. Adv Cancer Res. 2000;77:81–137.
Google Scholar
-
Rodrigues JM, Hassan M, Freiburghaus C, Eskelund CW, Geisler C, Räty R, et al. p53 is associated with high-risk and pinpoints TP53 missense mutations in mantle cell lymphoma. Br J Haematol. 2020;191:796–805.
Google Scholar
-
Nolan J, Murphy C, Dinneen K, Lee G, Higgins E, Bacon L, et al. p53 immunohistochemistry must be confirmed by TP53 next generation sequencing for accurate risk stratification of patients with mantle cell lymphoma. Leuk Lymphoma. 2022;63:3504–7.
Google Scholar
-
Kluin-Nelemans HC, Hoster E, Hermine O, Walewski J, Geisler CH, Trneny M, et al. Treatment of older patients with mantle cell lymphoma (MCL): long-term follow-up of the randomized European MCL elderly trial. Journal of Clinical Oncology. 2020;38:248–56.
Google Scholar
-
Hermine O, Jiang L, Walewski J, Bosly A, Szymczyk M, Thieblemont C, et al. Addition of high-dose cytarabine to immunochemotherapy before autologous stem-cell transplantation in patients aged 65 years or younger with mantle cell lymphoma (MCL Younger): a long-term follow-up of the randomized, open-label, phase 3 trial of the European Mantle Cell Lymphoma Network. Blood. 2021;138:380–380.
Google Scholar
-
Rule S, Dreyling M, Goy A, Hess G, Auer R, Kahl B, et al. Outcomes in 370 patients with mantle cell lymphoma treated with ibrutinib: a pooled analysis from three open-label studies. Br J Haematol. 2017;179:430–8.
Google Scholar
-
Rule S, Dreyling M, Goy A, Hess G, Auer R, Kahl B, et al. Ibrutinib for the treatment of relapsed/refractory mantle cell lymphoma: extended 3.5-year follow up from a pooled analysis. Haematologica. 2019;104:e211–e4.
Google Scholar
-
Dreyling M, Goy A, Hess G, Kahl BS, Hernández-Rivas J, Schuier N, et al. Long-term outcomes with ibrutinib treatment for patients with relapsed/refractory mantle cell lymphoma: a pooled analysis of 3 clinical trials with nearly 10 years of follow-up. Hemasphere. 2022;6:e712.
Google Scholar
-
Wang ML, Jurczak W, Jerkeman M, Trotman J, Zinzani PL, Belada D, et al. Ibrutinib plus bendamustine and rituximab in untreated mantle-cell lymphoma. N Engl J Med. 2022. https://doi.org/10.1056/NEJMoa2201817
Google Scholar
-
Dreyling M, Doorduijn JK, Gine E, Jerkeman M, Walewski J, Hutchings M, et al. Efficacy and safety of ibrutinib combined with standard first-line treatment or as substitute for autologous stem cell transplantation in younger patients with mantle cell lymphoma: results from the randomized triangle trial by the European MCL Network. Blood. 2022;140:1–3.
Google Scholar
-
Wang M, Munoz J, Goy A, Locke FL, Jacobson CA, Hill BT, et al. KTE-X19 CAR T-cell therapy in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2020;382:1331–42.
Google Scholar
-
Campo E, Jaffe ES, Cook JR, Quintanilla-Martinez L, Swerdlow SH, Anderson KC, et al. The international consensus classification of mature lymphoid neoplasms: a report from the Clinical Advisory Committee. Blood. 2022;140:1229–53.
Google Scholar
-
Alaggio R, Amador C, Anagnostopoulos I, Attygalle AD, Araujo IBDO, Berti E, et al. The 5th edition of the World Health Organization classification of haematolymphoid tumours: lymphoid neoplasms. Leukemia. 2022;36:1720–48.
Google Scholar
Acknowledgements
We thank the European MCL Network, the Italian Lymphoma Foundation (FIL) and the Nordic Lymphoma Group (NLG) for participation in this study.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
GS, LJ and EH wrote the manuscript, LJ performed the statistical analysis; AR and WK collected material; CS, MU, HK-N, OH, AE, ML, SF and MJ provided clinical data; AR and WK performed the immunohistochemistry analysis; OH, HK-N, EH and MD designed the study; and all authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
GS, LJ, OH, HCK-N, CS, MU, AR, WK, ML, AE and EH: None. MJ—Honoraria: Abbvie, Astra Zeneca, BMS, Genmab, Gilead, Pierre Fabre, Janssen, Roche. Research support: Abbvie, Astra Zeneca, BMS, Gilead, Janssen, Roche. SF—Research funding: Janssen, Morphosys, Gilead, Beigene. Consultancy: EusaPharma, Janssen, Sandoz, Abbvie. Advisory Board: EusaPharma, Janssen, Clinigen, Incyte, Italfarmaco. Speakers Honoraria: Janssen, EusaPharma, Servier, Gentili. MD—Research Support (institution): Abbvie, Bayer, BMS/Celgene, Gilead/Kite, Janssen, Roche. – Employee: -Major Stockholder: -Speakers Bureau: -Speakers Honoraria: Amgen, Astra Zeneca, Gilead/Kite, Janssen, Lilly, Novartis, Roche. – Scientific Advisory Board: Astra Zeneca, Beigene, BMS/Celgene, Gilead/Kite, Janssen, Lilly/Loxo, Novartis, Roche.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Presented in abstract form at the 61st American Society of Hematology (ASH 2019) Annual Meeting.
Supplementary information
Supplemental tables and figures
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
Reprints and Permissions
About this article
Cite this article
Scheubeck, G., Jiang, L., Hermine, O. et al. Clinical outcome of Mantle Cell Lymphoma patients with high-risk disease (high-risk MIPI-c or high p53 expression).
Leukemia (2023). https://doi.org/10.1038/s41375-023-01977-y
-
Received: 05 March 2023
-
Revised: 28 June 2023
-
Accepted: 17 July 2023
-
Published: 26 July 2023
-
DOI: https://doi.org/10.1038/s41375-023-01977-y