Blood
Dallas-Based Five Liters Tackles Blood Loss With Bioelectric Wearable Tech
[Image: DI; metamorworks/istockphoto]
Five Liters, a subsidiary of Dallas-based Spark Biomedical, is set to make strides in bioelectric wearable technology.
The new group is conducting “first in-human” studies to demonstrate the effectiveness of noninvasive wearable neurostimulation in reducing blood loss, particularly for individuals with blood disorders or undergoing surgical procedures.
The potential of neuromodulation to slow blood loss holds valuable applications in operating rooms, trauma scenes, and military missions, potentially saving lives and improving outcomes.
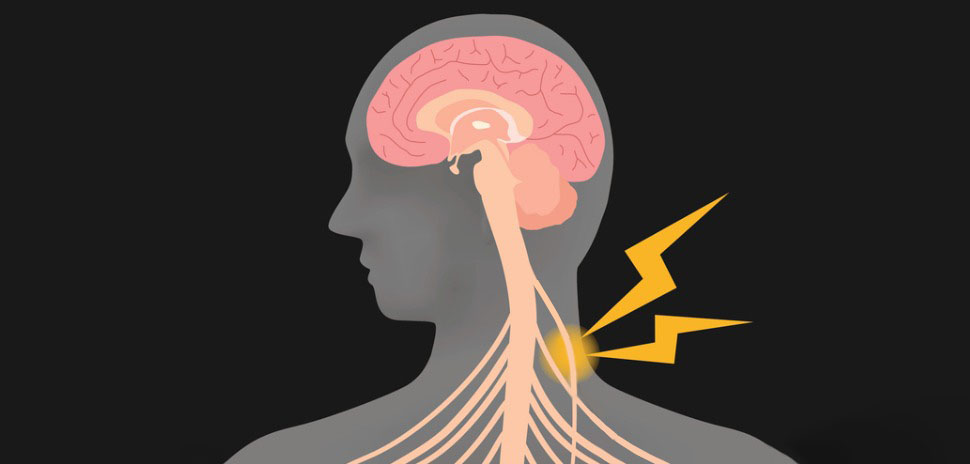
The human studies aim to validate findings recently published in Nature Communications last month. Vagus nerve stimulation reduced bleeding and improved clotting in male mice with hemophilia A, suggesting potential as a therapy for blood loss in people.
Spark Biomedical announces Five Liters
Five Liters is “perfectly positioned” to move the solution toward commercial application,” said Daniel Powell, the CEO of Spark Biomedical in a statement.

Daniel Powell [Image: DI; Photo: Spark Biomedical]
Dallas-based Spark Biomedical announced the formation of Five Liters in June as part of a collaboration with The Feinstein Institutes for Medical Research. Together, they hope to bring a wearable neurostimulation solution that reduces excessive blood loss to the market.
The Five Liters subsidiary allows Spark Biomedical to continue focusing on its device addressing opioid addiction while also advancing its work on a bioelectric medicine solution for reducing excessive blood loss, according to Powell.
Spark Biomedical, founded in 2015, recently received FDA clearance for its newest product, Sparrow Ascent, a wearable device designed to offer drug-free relief for people experiencing opioid withdrawal symptoms.
Powell is enthusiastic about working with the talented scientists at The Feinstein Institutes to bring the vision for blood loss solutions to reality, initially conceived over ten years ago. Spark Biomedical’s corporate infrastructure and patented neurostimulation platform—Transcutaneous Auricular Neurostimulation—can help move the project forward.
The collaboration combines the Feinstein Institutes’ years of research and patented concept of vagus nerve stimulation for blood loss reduction with Spark Biomedical’s FDA-approved therapy device expertise and experience in clinical trial management, FDA submission, market activation, and commercialization.
The product team, developed jointly by both companies, will build on the expertise of Feinstein Institutes’ experts Kevin J. Tracey, MD, and Jared M. Huston, MD, and their 15 years of intellectual property development, initially supported by the Defense Advanced Research Projects Agency (DARPA).
Studies conducted by the Feinstein Institutes have shown that electrical activation of neural pathways to the spleen can prepare the body for clotting in case of a wound, leading to a 50 percent reduction in bleeding time and volume in pre-clinical studies using VNS, according to the news release.
The Feinstein Institutes for Medical Research is home to the research institutes of Northwell Health, the largest healthcare provider and private employer in New York State. It comprises 50 research laboratories, conducts 3,000 clinical research studies, and is staffed by 5,000 researchers and staff members.
Dr. Tracey, president and CEO of The Feinstein Institutes, says the strategy behind the wearable neurostimulation solution has been “exhaustively” researched and tested.
“If successful, these trials should represent an important milestone in the history of this long-unsolved clinical situation,” Tracey said in a statement.
Targeting blood-clotting disorder
Should the study show positive results, Five Liters said it would prioritize a condition called Von Willebrand Disease (VWD). The disorder, affecting approximately 1 in every 100 people worldwide, causes excessive, and often, unpredictable, bleeding.
The Five Liters team believes its non-invasive, wearable neurostimulation tech could significantly alleviate these symptoms.
VWD manifests itself with varying degrees of bleeding tendency, such as excessive bleeding from injuries, surgical or dental procedures, persistent nosebleeds, heavy or prolonged menstrual bleeding, and bleeding during labor and delivery, the company said. Other symptoms include blood in urine or stool, easy bruising, and hematomas.
Navid Khodaparast, the Chief Science Officer of Spark and Five Liters, noted the severity of the situation. “Von Willebrand Disease is an inherited bleeding disorder in which blood does not clot properly, leading to bleeding events and joint problems.
Females with VWD, who represent nearly 80% of all patients, frequently endure heavy menstrual bleeding or menorrhagia, Khodaparast said.
“These monthly occurring bleeding events not only can take a toll on their physical health but can also affect their mental health, leading to significant depression and anxiety,” the CSO said.
Five Liters is set to launch a clinical trial later this year, focused on utilizing Transcutaneous Auricular Neurostimulation (tAN) therapy to alleviate menorrhagia in females with VWD, Khodaparast said.
There is no cure for VWD, but based on the promising pre-clinical work conducted by the Feinstein Institutes for Medical Research, the recently published Nature Communications article, and Five Liters’ affiliation with Spark Biomedical’s clinical research, the company said it is optimistic about the potential for a non-invasive, non-pharmacological treatment option.
Lance Murray contributed to this report.
![]()
Get on the list.
Dallas Innovates, every day.
Sign up to keep your eye on what’s new and next in Dallas-Fort Worth, every day.