Cancer and neoplasms
Prognosis of bloodstream infection with P aeruginosa
Introduction
Pseudomonas aeruginosa (PA) is an opportunistic pathogen with combined antibiotic resistance, multifactorial virulence, and dynamic over-adaptive capacity that is particularly difficult to eliminate from patients and is an important cause of nosocomial infections that can be life-threatening in critically ill and immunocompromised patients. The mortality rate of PA bloodstream infection (BSI) has been reported to be between 20% and 50%.1–3 Carbapenems are the most effective antibacterial agents against severe P. aeruginosa infections and are often used as a last resort in the treatment of bacterial infections. With the widespread and irrational use of carbapenem antibiotics, resistance to carbapenem antibiotics in PA is on the rise. Carbapenem-resistant Pseudomonas aeruginosa (CRPA) was listed by the World Health Organization in 2017 as a key priority pathogen for future research and development of novel antibiotics.4 According to the China Antimicrobial Surveillance Network (https://www.chinets.com/Data/GermYear), the resistance rates of imipenem and meropenem in PA infections were 30.7% and 25.8% in 2018 and 22.1% and 17.6% in 2022, respectively. The resistance rates of imipenem and meropenem in China have decreased slightly over the past 5 years, but remain at a high level. Patients with hematological malignancy (HM) appear to be more susceptible to CRPA infection due to primary immunodeficiency, neutropenia caused by hematopoietic stem cell transplantation or chemotherapy, and frequent exposure to broad-spectrum antibiotics.5,6 The mortality rate of patients with CRPA bloodstream infection has been reported to be as high as 44.12%and may be higher in patients with hematological malignancy, however, there are still few studies on CRPA bloodstream infection in patients with hematological malignancy.6 To clarify the risk factors affecting the prognosis of CRPA-BSI in patients with HM, 64 cases of HM patients combined with CRPA-BSI in our hospital from 2018 to 2022 were retrospectively analyzed and reported as follows.
Materials and Methods
Study Design and Data Collection
This was a retrospective study. During the study period, the population of CRPA isolates in our research limited to blood samples.HM patients with CRPA bloodstream infections occurring between January 2018 and December 2022 at the Cancer Hospital of Zhengzhou University were collected, and only the clinical data of the first occurrence were recorded for multiple isolations of CRPA from the same patient. Inclusion criteria: ① inpatients with a confirmed diagnosis of hematologic malignancy and complete clinical information; ② one or more positive blood cultures for CRPA and clinical evidence of the corresponding infection. Case data of 64 patients were collected from the hospital’s electronic medical record system, including age, gender, Body Mass Index (BMI), absolute neutrophil count, transaminases, bilirubin, whether transplantation was performed, primary disease, treatment modality, application of high-dose glucocorticosteroids within 90 days, central venous placement, indwelling urinary catheter, mechanical ventilation, Pitt bacteremia score (Pitt bacteremia score, PBS),7 whether septic shock, duration of hospitalization before BSI, carbapenem exposure before BSI, drug sensitivity results, and antibiotic therapy. To explore the risk factors for CRPA-BSI outcome, patients were divided into survival and non-survival groups according to whether they survived 28 days after the onset of CRPA-BSI.
Definition
The diagnosis of malignant hematologic diseases is based on the WHO Classification of hematological tumors.8,9 CRPA is defined as PA isolates that are resistant to at least one carbapenem (minimum inhibitory concentration of meropenem or imipenem ≥ 8 μg/mL). Bloodstream infection was defined as the presence of live bacteria in the bloodstream that resulted in clinical signs or symptoms of infection.10 Bacteremia occurrence was defined as the date of collection of blood cultures of the first CPRA-producing strain. Neutrophil deficiency (granulocyte deficiency) was defined as an absolute neutrophil count (ANC) of <0.5 × 109/L in peripheral blood, and severe granulocyte deficiency was defined as ANC <0.1 × 109/L. Septic shock was defined as persistent hypotension in a septic patient despite adequate fluid resuscitation and requiring vasopressor therapy to maintain mean arterial pressure at ≥ 65 mmHg.11 Empirical antimicrobial therapy was defined as antibiotic therapy received by the patient between the time blood cultures were drawn and drug sensitivity results were obtained. Definitive antimicrobial therapy was the antibiotic treatment given after the drug sensitivity results were reported.
Microbiological Methods
Blood cultures were performed using an automated BACTEC FX system (Becton Dickinson, Sparks, MD, USA), and the bacterial identification and drug sensitivity analyzer was a BD M-50, USA The method of drug sensitivity testing and determination of results strictly followed the American Clinical and Laboratory Standardization Institute (CLSI) Document M100 edition (2020) (http://em100.edaptivedocs.net/dashboard.aspx). Polymyxin was referred to the EU standard for drug sensitivity testing (https://www.eucast.org), and the rest of the folding points were referred to the requirements of the American Society for Clinical Laboratory Standardization M-100.
Statistical Methods
Continuous variables were expressed as mean ± standard deviation or median (range), and categorical variables were expressed as frequency and percentile. Cox proportional risk models were used to determine independent risk factors for 28-day mortality. Variables with P values ≤0.10 in the univariate analysis were included in the multifactorial Cox regression model. Results are reported as hazard ratios (HR) and 95% confidence intervals (CI). Statistical significance was considered at P < 0.05. Survival analysis was performed using the Kaplan-Meier method.12 All statistical analyses were performed in IBM SPSS 25.0, and P values <0.05 were statistically significant.
Ethical Statement
The study protocol was reviewed and approved by the Ethics Committee of the Cancer Hospital of Zhengzhou University (2023-188-001). Because patient data were analyzed anonymously and confidentiality was maintained, the requirement for patient consent was waived. The study was conducted in accordance with the Declaration of Helsinki.
Results
Clinical Characteristics of Patients
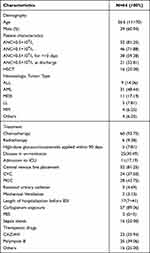
A total of 69 HM inpatients developed CRPA-BSI during the study period, of which 5 were excluded due to missing data, and a total of 64 patients were finally included in this study. Of the 64 HM inpatients with CRPA-BSI, 39 (60.94%) were male and 25 (39.06%) were female, with a median age of 36.5 (11–70) years. Among all patients, 52 (81.25%) had granulocyte deficiency (ANC <0.5×109/L), 46 (71.88%) had severe granulocyte deficiency (ANC 10 days, and 21 (32.81%) were still not free from granulocyte deficiency at discharge; 16 (25.00%) were hematopoietic stem cell transplantation (HSCT) patients and 48 were non-HSCT patients; 31 (48.44%) had acute myeloid leukemia (AML), 11 (17.19%) had myelodysplastic syndrome (MDS), 9 (14.06%) had acute lymphoblastic leukemia (ALL), 5 (7.81%) had lymphoma, 4 (6.25%) had multiple myeloma, and 4 (6.25%) were other hematologic malignancies; in terms of treatment modality, 60 (93.75%) received chemotherapy and 6 (9.38%) received radiotherapy; 5 (7.81%) applied high-dose hormones within 90 days before the onset of BSI; most patients underwent invasive procedures before CRPA BSI: 52 (81.25%) patients underwent central venous catheterization, of which 24 (37.50%) were central venous catheters (CVC) and 28 (43.75%) were central venous catheters (PICC) placed through peripheral veins; 3 (4.69%) had indwelling urinary catheters and 2 (3.13%) were mechanically ventilated; the median length of stay before the onset of BSI was 17 (7–41) days in 64 patients The median length of stay before BSI was 17 (7–41) days; 57 (89.06%) had carbapenem exposure before BSI; the median PBS score was 2 (0–5); and 16 (25.00%) had combined septic shock. 64 patients’ clinical characteristics are shown in Table 1.
|
Table 1 Clinical Characteristics of 64 HM Patients with CRPA-BSI |
Microbiological Characteristics
A total of 64 isolates were identified as CRPA. The isolation rate of CRPA ranged from 12.36% to 16.40% during the study period (Figure 1). The susceptibility data of the 64 CRPA isolates to antibiotics are shown in Table 2. Colistin and amikacin were the most active drugs, while amineptine and ceftazidime were the least active (Table 2). Because the susceptibility of ceftazidime-avibactam (CAZ/AVI) was not routinely tested in our institution, a total of eight cases were supplemented by the paper diffusion method (Kindy-Bauer, KB method) and all were found to be sensitive with a median inhibition circle diameter of 26 mm.
 |
Table 2 Susceptibility to Antibiotics of 64 CRPA Isolates |
|
Figure 1 Separation rate of CRPA during the study period. |
Patient Prognosis Analysis
Univariate analysis by Cox regression showed that ANC <0.5×109/L at discharge, admission to ICU, PBS ≥2 points, length of stay, and septic shock were associated with 28-day mortality, whereas treatment with polymyxin B or CAZ/AVI was associated with survival (p≤0.05); multivariate analysis showed that ANC <0.5×109/L at discharge (HR 0.039, 95% CI 0.006–0.258, p=0.001), admission to the ICU (HR 7.546, 95% CI 1.345–42.338, p= 0.022), the and PBS ≥2 score (HR 0.207, 95% CI 0.046 to 0.939, p = 0.041) were independent risk factors associated with 28-day mortality (Table 3).ROC curve analysis showed that a PBS threshold of 2 was a good predictor of mortality in patients with CRPA BSI HM, with an area under the curve of 0.800 (95% CI 0.668 to 0.911, p < 0.001), with a sensitivity of 78.1% and specificity of 75.0% (Figure 2). In Kaplan-Meier curve analysis, PBS ≥2 was associated with higher mortality (p < 0.001) (Figure 3A).
|
Table 3 Cox Regression Analysis Affecting Mortality of Carbapenem-Resistant Pseudomonas Aeruginosa Bloodstream Infections |
|
Figure 2 ROC curve of PBS score. |
 |
Figure 3 Survival curves of 64 patients with CRPA-BSI (A). Survival curves of patients with different PBS scores (B). Survival curves of patients in different drug treatment groups. |
Treatment and Regression
The overall 28-day mortality rate was 37.5% (24/64). 11 patients died before the return of the drug sensitivity results, 2 of whom received empirical treatment with a polymyxin B-based combination anti-infective regimen (1 received polymyxin B combined with tigecycline, 1 received polymyxin B combined with meropenem) and 9 with a carbapenem-based combination regimen. Of the 53 patients treated with definitive antibiotics, 23 received polymyxin B-based therapy, of which 18 were combinations: 8 in combination with carbapenems, 5 in combination with amikacin, and 5 in combination with piperacillin-tazobactam; 23 received ceftazidime avibactam-based therapy, of which 16 were single-agent applications, 3 in combination with aminoglutethimide, 2 in combination with amikacin, 2 in combination with carbapenems penicillin; 7 patients were treated with carbapenem + amikacin + piperacillin-tazobactam/ cefoperazone sulbactam. Survival analysis showed that patients treated with CAZ/AVI had a slightly higher survival rate than those treated with polymyxin B, but the difference was not statistically significant, but all were significantly better than those treated with other drugs (Figure 3B).
Discussion
In recent years, the rapid spread of carbapenem-resistant gram-negative bacteria (CRGNB) has become a major global public health problem. PA is a major global nosocomial pathogen13–15 with combined multifactorial virulence and multiple antibiotic resistance mechanisms, including intrinsic membrane permeability, efflux pump system, production of antibiotic-inactivating enzymes, and loss of pore protein function.4 In the United States, 10–30% of P. aeruginosa isolates are carbapenem resistant,16,17 and according to the Chinese Bacterial Resistance Surveillance Network (https://www.chinets.com/Data/GermYear), the resistance rates of imipenem and meropenem in PA infections in 2022 were 22.1% and 17.6%, respectively, with their high resistance rates limiting the options for antimicrobial therapy. It has been shown that carbapenem exposure is associated with CRPA BSI,6,18 and hematologic oncology patients are immunodeficient, often with granulocyte deficiency, have a high history of carbapenem exposure, and are therefore more susceptible to comorbid CRPA infections. Teelucksingh et al19 showed through a retrospective study that infectious shock, age, and PBS ≥4 were poor prognostic independent risk factors. However, studies on risk factors affecting the prognosis of CRPA-BSI in HM patients are still scarce, and in this study, 64 HM patients with combined CRPA-BSI were included, and Cox regression analysis showed that admission to the intensive care unit, higher PBS score, and granulocyte deficiency at discharge were independent risk factors for mortality at 28 days after BSI.
The Pitt bacteremia score (PBS) is a score widely used to assess the severity of acute infectious disease and was first used to predict morbidity and mortality in patients with PA BSI and has since been shown to have good predictive value for the risk of death in other gram-negative and positive bacteria and antibiotic-resistant bacteria and fungal BSI, ranging from 0–14, with PBS ≥4 usually suggesting increased critical illness and mortality.7,20–22 A recent retrospective study23 showed that higher PBS scores were independently associated with 28-day mortality after the development of CRKP bloodstream infection in HM patients, while few studies have been conducted regarding PBS in HM patients with CRPA BSI. The results of this year’s study showed that higher PBS was independently associated with 28-day mortality after the occurrence of CRPA BSI in HM patients, and ROC curve analysis showed that a critical PBS value of 2 was a good predictor of mortality in HM patients with CRPA BSI with a sensitivity of 78.1% and specificity of 75.0%.
Neutrophils are critical in the acute inflammatory response and the host’s defense against bacterial infection. Patients with granulocyte-deficient HM are at high risk of developing BSIs due to immunodeficiency, mucositis, central venous placement, and gastrointestinal bacterial colonization. A 14-year prospective longitudinal study in the UK showed a 3-fold higher incidence of bloodstream infections (BSI) in HM patients compared to other cancer patients24 and increased mortality from BSI caused by PA relative to BSI caused by S. aureus or other gram-negative bacteria.25 The results of a Meta-analysis showed a significant association between carbapenem resistance and poor clinical outcomes in PA bloodstream infections.3 Granulocyte deficiency was shown to be an independent predictor of mortality in patients with CRE BSI,26 and prolonged granulocyte deficiency (≥15 days) was independently associated with BSI death.27 In the present study, non-discharge from granulocyte deficiency at discharge were independent risk factors for mortality in HM patients who developed CRPA BSI. Therefore, more attention needs to be given to HM patients who develop granulocyte deficiency and longer duration of granulocyte deficiency in anti-infective therapy.
Data from the 2021 China Bacterial Resistance Surveillance Network showed that 5572 carbapenem-resistant strains of P. aeruginosa in China had a resistance rate of 1.5% to polymyxin B, 10.4% to amikacin, and 13.9% to ceftazidime-avibactam. Polymyxins remain a class of antibiotics available for many multidrug-resistant gram-negative bacteria almost 60 years after clinical approval, but they are nephrotoxic and neurotoxic.28 Whether polymyxin is used in monotherapy or combination therapy in the treatment of CRPA infections is controversial, and there is a lack of data comparing monotherapy and combination therapy in the treatment of CRPA infections. It has been suggested that monotherapy with polymyxins leads to bacterial regeneration and development of resistance during treatment29 and that combination therapy increases the likelihood of successful treatment of granulomatous patients with combined CRPA bloodstream infections, with in vitro pharmacovigilance confirming synergistic effects of carbapenems and polymyxins on most CRPA isolates and clinical studies showing lower mortality in patients treated with this combination.30,31 However, in a subgroup analysis of CRPA-infected patients, both the AIDA and OVERCOME trials showed no significant difference between mucilage monotherapy and the combination regimen of mucilage plus meropenem in terms of 28-day mortality.32 With increasing use, resistance to polymyxin in P. aeruginosa has emerged,33,34 and further optimization of its clinical use is needed to minimize the development of resistance.
Ceftazidime-avibactam is a novel β-lactamase inhibitor4 with strong antibacterial activity against PA,35 suggesting that CAZ/AVI may be an alternative treatment option for CRPA.36,37 A retrospective study included 136 patients with CRPA infection, 51 receiving CAZ/AVI monotherapy and 85 receiving polymyxin-based combination therapy, and showed that patients treated with CAZ/AVI had significantly lower 14-day mortality, 30-day mortality, and in-hospital mortality than those treated with polymyxin B. The CAZ/AVI group had a significantly higher bacterial clearance rate than the polymyxin B group.38 Our expert consensus on PA lower respiratory tract infections also states that for CRPA infections, ceftazidime avibactam can be used as a first-line treatment option when sensitivity to it is confirmed by drug sensitivity.39 The 28-day mortality rate of 64 patients in this study was 37.5%, of which 11 patients died before the return of the drug sensitivity. 2 of them received empirical treatment with a polymyxin B-based combination anti-infective regimen (1 received polymyxin B combined with tigecycline, 1 received polymyxin B combined with meropenem) and 9 received a carbapenem-based combination regimen (imipenem-cilastatin/ meropenem±amikacin±aminotransol). Survival analysis showed that patients treated with CAZ/AVI had a slightly higher survival rate than those treated with polymyxin B, but the difference was not statistically significant, but all were significantly better than those treated with other drugs. Thus early anti-infective treatment with ceftazidime-avibactam or polymyxin B may improve the clinical prognosis of patients.
The current study had several limitations. It was conducted in a specialized tumor hospital and only included HM patients. The findings may not be applicable to other settings or patients. Furthermore, not all patients underwent phenotypic screening and detection. Lastly, the small number of patients in the study may have influenced the capacity of the analysis to identify risk factors and outcomes.
Data Sharing Statement
The datasets generated during the current study are available from the corresponding author on reasonable request.
Ethics Declarations
The study was approved by the Ethics Committee of the Affiliated Cancer Hospital of Zhengzhou University, China.
Acknowledgments
We would like to acknowledge the nursing staff, transplant coordinators, and Laboratory Medicine staff who greatly contributed to this work.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported in part by Henan medical science and technology project LHGJ20220186, 232102310201.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Recio R, Mancheño M, Viedma E, et al. Predictors of mortality in bloodstream infections caused by Pseudomonas aeruginosa and impact of antimicrobial resistance and bacterial virulence. Antimicrob Agents Chemother. 2020;64(2). doi:10.1128/AAC.01759-19
2. Lodise TP, Bassetti M, Ferrer R, et al. All-cause mortality rates in adults with carbapenem-resistant Gram-negative bacterial infections: a comprehensive review of pathogen-focused, prospective, randomized, interventional clinical studies. Expert Rev Anti Infect Ther. 2022;20(5):707–719. doi:10.1080/14787210.2022.2020099
3. Zhang Y, Chen X-L, Huang A-W, et al. Mortality attributable to carbapenem-resistant Pseudomonas aeruginosa bacteremia: a meta-analysis of cohort studies. Emerg Microbes Infect. 2016;5(3):e27. doi:10.1038/emi.2016.22
4. Tacconelli E, Carrara E, Savoldi A, et al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis. 2018;18(3):318–327. doi:10.1016/S1473-3099(17)30753-3
5. Satlin MJ, Jenkins SG, Walsh TJ. The global challenge of carbapenem-resistant Enterobacteriaceae in transplant recipients and patients with hematologic malignancies. Clin Infect Dis. 2014;58(9):1274–1283. doi:10.1093/cid/ciu052
6. Wei X, Li L, Li M, et al. Risk factors and outcomes of patients with carbapenem-resistant Pseudomonas aeruginosa bloodstream infection. Infect Drug Resist. 2023;16:337–346. doi:10.2147/IDR.S396428
7. Rhee JY, Kwon KT, Ki HK, et al. Scoring systems for prediction of mortality in patients with intensive care unit-acquired sepsis: a comparison of the Pitt bacteremia score and the Acute Physiology and Chronic Health Evaluation II scoring systems. Shock. 2009;31(2):146–150. doi:10.1097/SHK.0b013e318182f98f
8. Alaggio R, Amador C, Anagnostopoulos I, et al. The 5th edition of the World Health Organization classification of haematolymphoid tumours: lymphoid neoplasms. Leukemia. 2022;36(7):1720–1748. doi:10.1038/s41375-022-01620-2
9. Khoury JD, Solary E, Abla O, et al. The 5th edition of the World Health Organization classification of haematolymphoid tumours: myeloid and histiocytic/dendritic neoplasms. Leukemia. 2022;36(7):1703–1719. doi:10.1038/s41375-022-01613-1
10. Yangco B. CDC definitions for nosocomial infections. Am J Infect Control. 1989;17(1):42–43. doi:10.1016/S0196-6553(89)80013-6
11. Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS international sepsis definitions conference. Crit Care Med. 2003;31(4):1250–1256. doi:10.1097/01.CCM.0000050454.01978.3B
12. George B, Seals S, Aban I. Survival analysis and regression models. J Nucl Cardiol. 2014;21(4):686–694. doi:10.1007/s12350-014-9908-2
13. Horcajada JP, Montero M, Oliver A, et al. Epidemiology and treatment of multidrug-resistant and extensively drug-resistant Pseudomonas aeruginosa infections. Clin Microbiol Rev. 2019;32(4). doi:10.1128/CMR.00031-19
14. Wang MG, Liu Z-Y, Liao X-P, et al. Retrospective data insight into the global distribution of carbapenemase-producing Pseudomonas aeruginosa. Antibiotics. 2021;10(5):548. doi:10.3390/antibiotics10050548
15. Al-Orphaly M, Hadi HA, Eltayeb FK, et al. Epidemiology of multidrug-resistant Pseudomonas aeruginosa in the Middle East and North Africa Region. mSphere. 2021;6(3). doi:10.1128/mSphere.00202-21
16. Almarzoky Abuhussain SS, Sutherland CA, Nicolau DP. In vitro potency of antipseudomonal β-lactams against blood and respiratory isolates of P. aeruginosa collected from US hospitals. J Thorac Dis. 2019;11(5):1896–1902. doi:10.21037/jtd.2019.05.13
17. Woodworth KR, Walters MS, Weiner LM, et al. Vital signs: containment of novel multidrug-resistant organisms and resistance mechanisms — United States, 2006–2017. MMWR Morb Mortal Wkly Rep. 2018;67(13):396–401. doi:10.15585/mmwr.mm6713e1
18. Chaves L, Tomich LM, Salomão M, et al. High mortality of bloodstream infection outbreak caused by carbapenem-resistant P. aeruginosa producing SPM-1 in a bone marrow transplant unit. J Med Microbiol. 2017;66(12):1722–1729. doi:10.1099/jmm.0.000631
19. Teelucksingh K, Shaw E. Clinical characteristics, appropriateness of empiric antibiotic therapy, and outcome of Pseudomonas aeruginosa bacteremia across multiple community hospitals. Eur J Clin Microbiol Infect Dis. 2022;41(1):53–62. doi:10.1007/s10096-021-04342-y
20. Lee CC, Wang J-L, Lee C-H, et al. Age-related trends in adults with community-onset bacteremia. Antimicrob Agents Chemother. 2017;61(12). doi:10.1128/AAC.01050-17
21. Liu KS, Tong Y-S, Lee M-T, et al. Risk factors of 30-day all-cause mortality in patients with carbapenem-resistant Klebsiella pneumoniae bloodstream infection. J Pers Med. 2021;11(7):616. doi:10.3390/jpm11070616
22. Chen L, Han X, Li Y, et al. Assessment of mortality-related risk factors and effective antimicrobial regimens for treatment of bloodstream infections caused by carbapenem-resistant enterobacterales. Antimicrob Agents Chemother. 2021;65(9):e0069821. doi:10.1128/AAC.00698-21
23. Meng H, Han L, Niu M, et al. Risk factors for mortality and outcomes in hematological malignancy patients with carbapenem-resistant Klebsiella pneumoniae bloodstream infections. Infect Drug Resist. 2022;15:4241–4251. doi:10.2147/IDR.S374904
24. Schelenz S, Nwaka D, Hunter PR. Longitudinal surveillance of bacteraemia in haematology and oncology patients at a UK cancer centre and the impact of ciprofloxacin use on antimicrobial resistance. J Antimicrob Chemother. 2013;68(6):1431–1438. doi:10.1093/jac/dkt002
25. Thaden JT, Park LP, Maskarinec SA, et al. Results from a 13-year prospective cohort study show increased mortality associated with bloodstream infections caused by Pseudomonas aeruginosa compared to other bacteria. Antimicrob Agents Chemother. 2017;61(6). doi:10.1128/AAC.02671-16
26. Li C, Li Y, Zhao Z, et al. Treatment options and clinical outcomes for carbapenem-resistant Enterobacteriaceae bloodstream infection in a Chinese university hospital. J Infect Public Health. 2019;12(1):26–31. doi:10.1016/j.jiph.2018.08.002
27. Wang L, Wang Y, Fan X, et al. Prevalence of resistant gram-negative bacilli in bloodstream infection in febrile neutropenia patients undergoing hematopoietic stem cell transplantation: a single center retrospective cohort study. Medicine. 2015;94(45):e1931. doi:10.1097/MD.0000000000001931
28. Roch M, Sierra R, Andrey DO. Antibiotic heteroresistance in ESKAPE pathogens, from bench to bedside. Clin Microbiol Infect. 2023;29(3):320–325. doi:10.1016/j.cmi.2022.10.018
29. Papst L, Beović B, Pulcini C, et al. Antibiotic treatment of infections caused by carbapenem-resistant Gram-negative bacilli: an international ESCMID cross-sectional survey among infectious diseases specialists practicing in large hospitals. Clin Microbiol Infect. 2018;24(10):1070–1076. doi:10.1016/j.cmi.2018.01.015
30. Zusman O, Avni T, Leibovici L, et al. Systematic review and meta-analysis of in vitro synergy of polymyxins and carbapenems. Antimicrob Agents Chemother. 2013;57(10):5104–5111. doi:10.1128/AAC.01230-13
31. Ramos JF, Leite G, Martins RCR, et al. Clinical outcome from hematopoietic cell transplant patients with bloodstream infection caused by carbapenem-resistant P. aeruginosa and the impact of antimicrobial combination in vitro. Eur J Clin Microbiol Infect Dis. 2022;41(2):313–317. doi:10.1007/s10096-021-04361-9
32. Paul M, Daikos GL, Durante-Mangoni E, et al. Colistin alone versus colistin plus meropenem for treatment of severe infections caused by carbapenem-resistant Gram-negative bacteria: an open-label, randomised controlled trial. Lancet Infect Dis. 2018;18(4):391–400. doi:10.1016/S1473-3099(18)30099-9
33. Landman D, Bratu S, Alam M, Quale J. Citywide emergence of Pseudomonas aeruginosa strains with reduced susceptibility to polymyxin B. J Antimicrob Chemother. 2005;55(6):954–957. doi:10.1093/jac/dki153
34. Gales AC, Jones RN, Sader HS. Global assessment of the antimicrobial activity of polymyxin B against 54 731 clinical isolates of Gram-negative bacilli: report from the SENTRY antimicrobial surveillance programme (2001–2004). Clin Microbiol Infect. 2006;12(4):315–321. doi:10.1111/j.1469-0691.2005.01351.x
35. Yin D, Wu S, Yang Y, et al. Results from the China Antimicrobial Surveillance Network (CHINET) in 2017 of the in vitro activities of ceftazidime-avibactam and ceftolozane-tazobactam against clinical isolates of Enterobacteriaceae and Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2019;63(4). doi:10.1128/AAC.02431-18
36. Nichols WW, de Jonge BLM, Kazmierczak KM, et al. In vitro susceptibility of global surveillance isolates of Pseudomonas aeruginosa to ceftazidime-avibactam (INFORM 2012 to 2014). Antimicrob Agents Chemother. 2016;60(8):4743–4749. doi:10.1128/AAC.00220-16
37. Testa R, Cantón R, Giani T, et al. In vitro activity of ceftazidime, ceftaroline and aztreonam alone and in combination with avibactam against European Gram-negative and Gram-positive clinical isolates. Int J Antimicrob Agents. 2015;45(6):641–646. doi:10.1016/j.ijantimicag.2014.12.033
38. Chen J, Liang Q, Chen X, et al. Ceftazidime/avibactam versus polymyxin B in the challenge of carbapenem-resistant Pseudomonas aeruginosa infection. Infect Drug Resist. 2022;15:655–667. doi:10.2147/IDR.S350976
39. Shi Y. [Chinese expert consensus on the management of lower respiratory tract infections of Pseudomonas aeruginosa in adults(2022)]. Zhonghua Jie He He Hu Xi Za Zhi. 2022;45(8):739–752. Chinese. doi:10.3760/cma.j.cn112147-20220407-00290