Blood
Seroprevalence of leptospirosis among blood donors in an endemic area
Abstract
Thailand is known to be endemic for leptospirosis. This bacterium may pose a potential risk to transfusion safety. This study was a cross-sectional study examining the seroprevalence of leptospirosis among Thai blood donors. A total of 1053 serum specimens collected from blood donors residing in 5 regions of Thailand during March to September 2020 were included in this study. All samples were tested for the presence of antibodies to 22 leptospiral serovars using the microscopic agglutination test (MAT) and anti-Leptospira IgG antibodies using commercially available enzyme immunoassay. We found no evidence of recent exposure to Leptospira spp. in sera of healthy Thai blood donors by MAT, including those in higher-risk areas. However, in this same group, we did find small numbers of past exposure (1.7%) to Leptospira spp. by IgG ELISA. According to the findings of this study, there is currently no evidence for implementing new blood banking procedures to identify possible carriers in Thailand, however these should be continually monitored and revised according to the infectious disease burden in each country. It should be noted that there was a difference in the occupation rate between the general population reported in Thailand and blood donors in this study; it may not reflect the actual situation in the country.
Introduction
Leptospirosis is a neglected tropical disease and one of the most common bacterial zoonoses worldwide, causing an estimated 1.03 million cases and 58,900 deaths annually1. The disease is endemic in many tropical countries and often has a seasonal distribution, increasing with heavy rain and higher temperatures2.
Leptospirosis is a major public health problem in Thailand, with an average annual incidence rate of 3.19 cases per 100,000 population and a case fatality rate of 0.04 cases per 100,000 population from 2020 to 20223. Most leptospirosis-confirmed cases occur in the northeast and south regions of the country3, with the highest incidence during the rainy season. Most reported cases are in agricultural workers such as rice cultivators, who are likely to be exposed to contaminated environments during their daily activities4,5,6. Various mammals such as rodents, livestock, and domestic pet act as reservoir hosts, and infections are acquired through infected urine or a contaminated environmental source, typically floodwater7,8.
The causative agents of leptospirosis are spirochetes of the genus Leptospira of which there are more than 60 species have been described for the entire genus Leptospira, including 17 pathogenic species9. Clinical infection presents in a wide variety of manifestations ranging from a mild influenza like illness to serious illness including Weil’s syndrome (characterized by jaundice, renal failure and myocarditis), meningitis and pulmonary haemorrhage10. The infection has an incubation period of 5–14 days with a range of 2–30 days. Symptoms mimic many other diseases such as dengue, influenza and viral haemorrhagic diseases8.
Many infectious disease agents pose a risk to transfusion safety11. The risk of transfusion-transmitted infections is lower than previously due to strict regulations on the use of blood products, including risk-based assessments for donors and screening of blood products12,13. However, the transmission of infectious diseases through blood products is still a major concern worldwide, and as a high-risk area, it is possible that leptospirosis could pose a risk to transfusion safety in Thailand. There is evidence that transmission of Leptospira by blood transfusion is possible14,15. One case has been reported in India, indicating that blood transfusion from an asymptomatic carrier donor can result in the transmission of leptospirosis in the recipient16.
This is a cross sectional, descriptive study of leptospirosis seroprevalence in Thailand. It has relevance since leptospirosis is an important infectious disease in tropical regions such as Asia, and clinical diagnosis may be missed in areas where dengue or influenza are also endemic. Blood transfusion poses an important risk for infectious disease transmission as has been seen with HIV, malaria and hepatitis in regions where they are endemic, and it is justified to screen for common diseases in blood banks given the risk of transmission. We therefore examined leptospirosis seroprevalence rates among a large cohort of healthy Thai blood donors. This research adds important evidence to inform decision making regarding transfusion transmitted infections.
Results
Demographic data
A total of 1053 serum specimens from 5 regions of Thailand were included in this study. The demographic characteristics of the studied population are presented in Table 1. Overall, 50% of the samples were from male donors, and the median age was 35 years (range, 18–66 years). They were equally collected from 5 provinces representing five regions of Thailand. Most donors were office workers (35.9%) with no underlying disease.
Seroprevalence of leptospirosis among blood donors
None of the 1053 serum samples had antibody titers suggestive of a recent infection by MAT. However, 18 donors (1.7%) were positive for anti-Leptospira IgG antibodies by ELISA. The median age of donors with Leptospira IgG ELISA-positive was 34 years (range 27–49 years), and 66.7% were male.
There were no statistically significant differences in the gender (p = 0.155), age (p = 0.458), living area (p = 0.624), and occupation (p = 0.074) of the individuals between Leptospira IgG ELISA-positive and Leptospira IgG ELISA-negative. The detailed information of donors who were Leptospira IgG ELISA reactive was shown in Table 2.
Factors associated with Leptospira IgG-positive ELISA
Logistic regression analysis was used to assess the factor associated with the presence of anti-Leptospira IgG antibodies. However, the result indicated that there was no significant effect of age, gender, BMI, or region on the rates of Leptospira IgG ELISA-positive (p < 0.05).
Discussion
Thailand is known to be endemic for leptospirosis. This bacterium may pose a potential risk to transfusion safety, especially in acute asymptomatic cases which may favour transfusion-related transmission. In this study, we found no evidence of recent exposure to Leptospira spp. in sera of healthy Thai blood donors by MAT, including those in higher-risk areas. However, in this same group, we did find small numbers of past exposure (1.7%) to Leptospira spp. by IgG ELISA.
There are possible reasons for the inconsistent result between MAT and ELISA from our findings. MAT is one of the gold standard methods for diagnosing leptospirosis which detects serovar-specific antibodies17, however there are limitations in using this method. Previous studies (including one in Thailand) have demonstrated low sensitivity of MAT as compared to MAT with culture18. Indeed, research has demonstrated that regional Leptospira serovar-specific IgG ELISA is superior to MAT in diagnosing leptospirosis19. There are several potential reasons for the low performance of MAT, including that it may take several weeks after infection for specific antibodies against the bacteria to reach detectable levels20. In addition, MAT uses a live panel of Leptospira representing the main serovars and for proper performance of the test, an optimized panel of antigens must be selected21. The use of MAT therefore requires an accurate knowledge of local circulating serovars with regular surveillance to maintain a complete panel of relevant antigens22. It is therefore possible that in our study the panel of antigens used in the MAT did not cover locally circulating serovars in Thailand. In addition, Leptospira contamination of donated blood occurs when the donor is in the early stages of infection. At this time, the donor has not yet produced sufficient antibodies and may not be detected using MAT. Regarding IgG ELISA, we used a commercially available kit that uses a native membrane extract from Leptospira biflexa with genus-specific antigens. Therefore, the kit can detect IgG antibodies directed against all Leptospira spp including pathogenic Leptospira. For this reason, the IgG ELISA detection rate may be higher than the MAT. However, information regarding the exact protein of L. biflexa that the kit used as an antigen was not provided by the company.
Data from the Thailand Ministry of Public Health from 2003 to 2012 indicated that the incidence rates of leptospirosis were highest in the northeastern region23. However, there has been a recent shift with a higher incidence of leptospirosis reported in the southern region since 20203. There are significant environmental and cultural differences between the northeastern and southern regions of Thailand including in people’s lifestyles, housing, and ecology24. Northeast Thailand is a high-flat plain and relatively low humidity. Households frequently share various water sources for agricultural usage. Most of the study population in this region worked in fields frequently exposed to animals and environmental bodies of water, like rice paddy fields. Southern Thailand is high humidity and heavy rains, mainly covered in tropical rainforests. Instead of working in livestock farming or other jobs involving animals, most people work on rubber or palm plantations24. However, Leptospira IgG detection rates in our cohort of blood donors did not vary by area.
Seroprevalence data of leptospirosis in the Thai general population are limited. The first nationwide leptospirosis seroprevalence study (using agglutination tests) was conducted in 1966 on nonfebrile adult patients in hospitals across Thailand25. This study reported positive agglutination reactions for various serotypes of leptospirosis in 22–35% of the 3746 people examined25. A second study (using Leptospira IgG ELISA) was conducted on repository serum specimens obtained from young Thai men entering the Royal Thai Army without suspicious symptoms during 2007–2008 and found an overall seroprevalence rate of 28%26. Recently, Chadsuthi et al. used MAT to analyze Leptospira seroprevalence in 1990 human serum samples under suspicion of leptospirosis collected from 5 regions of Thailand between 2010 and 2015, among these 23.7% were found seropositive27.
We used a health blood donor population as a proxy for the general population, and there is a risk that our included population may not be the population at high risk of leptospirosis. Blood donor based serosurveillance has long been used as a powerful tool and cost-effective strategy providing insights on past emerging infectious threats such as West Nile, dengue, chikungunya, Zika and more recently COVID-1928,29,30,31,32,33,34. However, care must always be taken when extrapolating data from donor seroprevalence studies to the general population, as blood donor demographics will be different to those of the general population.
In our study we looked at a cohort of blood donors, a population that represents a potential source of transmission of leptospirosis through blood transfusion. This risk is low, with only one such case previously described in India16, however the risk of blood borne transmission from infectious diseases such as leptospirosis that can present asymptomatically (meaning that carrier status is difficult to detect) necessities strict enforcement of blood donors screening practices. This is particularly relevant in areas where adequate screening of blood products may not be performed.
Fortunately, Thailand has a strong haemovigiliance system in place adhering strictly to standard WHO guidelines including implementing a strategic plan, relevant legislation and regular inspections of facilities. The Thailand Blood Centre works with the Thai Red Cross Society in providing transfusion services across the country through a National Blood Center, 12 Regional Blood Centers, 166 blood service branches and a plasma fractionation center35. In 2010, the Thailand National Blood Centre in cooperation with the Ministry of Public Health created the National Blood Policy which aimed to provide safe blood for patients in accordance with the principles of the WHO; namely by recruiting blood donations from a low-risk population, screening blood donors, standardised testing of all units of blood, and conducting a compatibility test for ensuring safe transfusion36. For the safety of donors and recipients of blood transfusion, all donors need to answer a questionnaire which includes general health and conditions that might increase infection risk36. The standard infections screened for in Thailand include HIV 1/2 and HIV p24 antigen, HBsAg, anti-HCV ELISA, Rapid Plasma Reagin (RPR) or Treponema Pallidum Hemagglutination Asssay (TPHA) and syphilis antibodies36. Additionally, NAT is used for HIV, HBV, and HCV in the negative serology screening test unit as a sequential test. Leptospira is not considered in screening, as per global haemovigilance standards. Our findings of low prevalence of serum leptospirosis among blood donors from highly endemic areas therefore provide support for current Thai blood donor screening practices.
In this study, we could not identify factors associated with past exposure to Leptospira spp. which may be related to the small number of IgG positive cases. An estimated leptospirosis seroprevalence of 28% based on a previous study in young men entering the Thai Army was used for our sample size calculations26.
There are several strengths to our study. Whilst there has been similar research conducted in other countries, this is the first attempt at quantifying the seroprevalence of leptospirosis among a healthy blood donor population in Thailand, an endemic area for leptospirosis. Our data included a large sample size of 1053 serum specimens which is one of the largest cohorts of blood donors tested for seroprevalence of leptospirosis that we have found in the published literature14,37,38,39 as illustrated in Table 3.
Our study does have several limitations. Firstly, we were not able to collect data on exposure history such as direct contact with body fluids or organs of infected animals, as well as routes of infection through additional sources such as indirectly through contaminated soil or water. Secondly, the present study did not perform a qPCR test. Therefore, we might miss some acute leptospirosis infection cases and further studies are needed on using a qPCR-based molecular method as a complementary test to MAT and ELISA. Finally, leptospiral infections are typically high-risk among outdoor workers, especially agricultural workers; however, in this study, we focused on the seroprevalence of leptospirosis in a blood donor population to assess the potential risk to transfusion safety. We used a random sampling method that randomly selects participants from the blood donor population even if they may not be a high-risk group. Therefore, the results should be interpreted with a degree of caution. Regarding occupational backgrounds, one of the largest studies which included 30,115 samples obtained from the National Blood Center in Bangkok and two Regional Blood Centers in Lop Buri province and Chon Buri province indicated that most of the donors were private sector workers (63%) followed by government workers (13%) and students (12%)41. Agricultural workers were classified as ‘other’, accounted for less than 12% of included donors41. However, it should be noted that the occupation trend in blood donors may be different from the general Thai population41.
Overall, our findings provide support for the appropriateness and effectiveness of current relevant Thailand donor selection policies and suggest that, even in areas with a relatively high incidence of leptospirosis, this bacterium does not currently seem to be a primary concern for blood services.
Conclusions
Leptospirosis remains a highly endemic infectious disease in Thailand. Current blood donor screening programs do not include detection of Leptospira spp. The seroprevalence of leptospirosis among a cohort of healthy Thai blood donors is low with no donors demonstrating acute infection with leptospirosis, and small numbers (1.7%) demonstrating evidence of past exposure. According to the findings of this study, there is currently no evidence for implementing new blood banking procedures to identify possible carriers in Thailand, however these should be continually monitored and revised according to the infectious disease burden in each country. It should be noted that there was a difference in the occupation rate between the general population reported in Thailand and blood donors in this study; it may not reflect the actual situation in the country.
Methods
Study design and population
The study was a cross-sectional study examining the seroprevalence of leptospirosis among Thai blood donors. An initial sample size of 861 for the study was determined, based on an estimated leptospirosis seroprevalence of 28%26 at a confidence level of 95% and precision of 3%. A total of 1053 samples were tested, including indeterminate and invalid results.
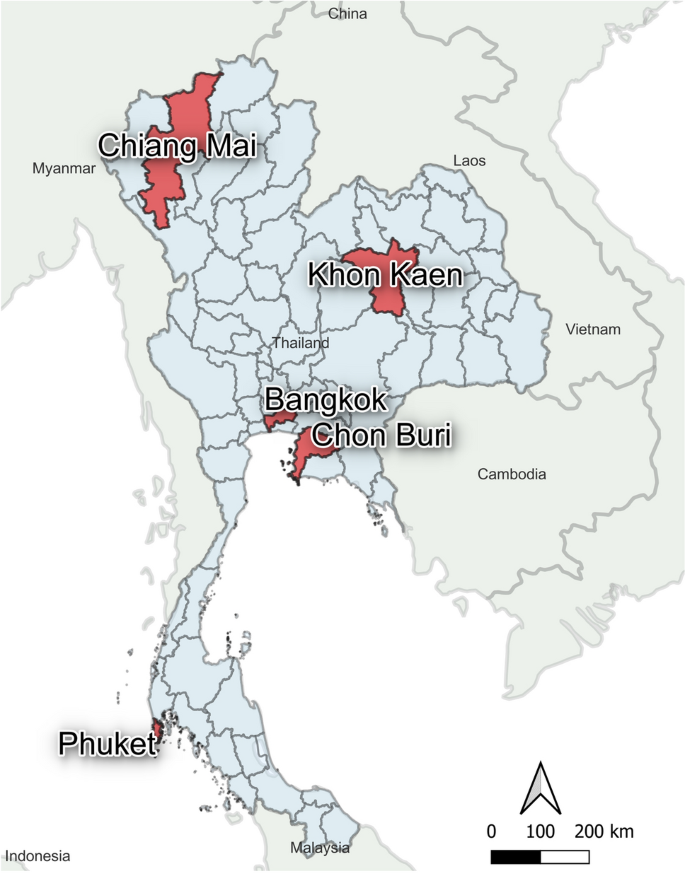
Donor blood samples were randomly selected from healthy volunteers with no past medical history of any significant infection or illness. All volunteers were confirmed to be healthy by physical examination. Blood samples were obtained from the National Blood Center in Bangkok (N = 218) and four regional blood centers located in Chiang Mai (N = 201), Khon Kaen (201), Chon Buri (N = 233), and Phuket province (N = 200) between March to September 2020 as shown in Fig. 1. We aimed to include similar numbers of volunteers from each regional blood bank. Participants were approached and asked to be a volunteer for the study when they arrived at the blood bank. All participants gave written informed consent, and the study was conducted according to the Helsinki Declaration and Good Clinical Practice guidelines. The study protocol was approved by the Research Ethics Committee of the National Blood Center, the Thai Red Cross (No. NBC 13/2020). Whole blood samples were collected into a serum collection tube (red topped tube) and sat in an undisturbed upright position for at least 15–30 min at room temperature to allow the blood to clot. The tube was centrifuged for 10 min at 3000 RPM. The liquid component (serum) was immediately transferred into a clean polypropylene tube and stored in aliquots at – 80 °C for further analysis. Demographic data on blood donors was obtained for all samples.
The distribution of blood centers involved in the study. 5 blood centers from 5 provinces in Thailand were enrolled in the present study (red colored regions in the map). The map was generated by geographical information system QGIS 3.28 software.
Detection of anti-Leptospira IgG antibody
All ssoftware…ples were analyzed for anti-Leptospira IgG antibodies using commercially available enzyme immunoassay (SERION ELISA classic Leptospira IgG, catalog number ESR125G, Institut Virion/Serion GmbH, Warburg, Germany).
The kit is designed to detect IgG antibodies against all Leptospira species. The kit uses a native membrane extract from Leptospira biflexa with genus-specific antigens. It is able to detect human antibodies against Leptospira spp, including all pathogenic Leptospira. The kit was used in several seroprevalence studies42,43,44. The assays were performed following the manufacturer’s instructions.
Briefly, 10 μL of serum sample was diluted with 1,000 μL of dilution buffer and mixed thoroughly to ensure the solutions were homogenous. Then 100 μL of the diluted sample or ready-to-use controls were added to wells of the microtiter test strips and incubated at 37 °C for 60 min in a moist chamber. After incubation, wells were washed four times with 300 μL washing solution, and 100 μL of ready-to-use anti-human-IgG from goat (polyclonal), conjugated to alkaline phosphatase, was added to the wells and incubated at 37 °C for 30 min in the moist chamber. Following the second incubation, all wells were washed four times with 300 μL washing solution, and 100 μL of ready-to-use para-nitrophenylphosphate substrate was added, followed by incubation at 37 °C for 30 min in the moist chamber. Finally, 100 μL of 1.2 N sodium hydroxide-stopping solution was added to each well, and the microtest plate was gently shaken to mix. The optical density (OD) was read within 60 min at 405 nm against substrate blank. Qualitative analysis was evaluated for IgG antibodies. The cut-off value was calculated by multiplying the mean value of the measured standard-OD with the numerical data of the certificate of quality control. The range of the cutoff was in between 0.497 and 0.627. According to the kit’s insert, the assay used has a sensitivity of 94.7% and a specificity of > 99%. Positive and negative controls were included in each run.
The microscopic agglutination test (MAT)
All samples were also tested by microscopic agglutination test (MAT) to detect prior Leptospira infection using a panel of 24 reference serovars including Australis, Autumnalis, Ballum, Bataviae, Canicola, Cellidoni, Cynopteri, Djasiman, Grippotyphosa, Hebdomadis, Icterohaemorrhagiae, Javanica, Louisaina, Manhao, Mini, Panama, Pomona, Pyrogenes, Ranarum, Sarmin, Sejroe, Shermani, Tarasovi, Semaranga. Sera were initially screened at 1:50 dilution and those showing 50% or more agglutination under a dark-field microscope were then serially diluted further to determine a titer endpoint. Samples with titers ≥ 1:50 were considered as past infection, while a titer of 1:400 or higher was used to define recent infection. This cut-off point has been used in other seroprevalence studies37,45,46.
Statistical analysis
Statistical analysis was performed with IBM SPSS Statistics Version 22 (SPSS, Chicago, IL). Categorical data were expressed as numbers with percentages. Continuous variables are reported as mean and standard deviation in the case of a normal distribution and as a median and interquartile range in the case of non-normal distribution. We used a univariate logistic regression analysis to assess the relationship between gender, age, BMI, and living area with anti-Leptospira antibodies. If the p-value is lower than 0.05, the result is considered significant.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
-
Costa, F. et al. Global morbidity and mortality of leptospirosis: A systematic review. PLoS Negl. Trop. Dis. 9, e0003898. https://doi.org/10.1371/journal.pntd.0003898 (2015).
Google Scholar
-
World Health Organization Regional Office for South-East Asia. Leptospirosis: Fact sheet. https://apps.who.int/iris/handle/10665/205437 (2009).
-
Bureau of Epidemiology, Department of Disease Control & Ministry of Public Health. Leptospirosis [in Thai]. http://doe.moph.go.th/surdata/disease.php?ds=43 (2021).
-
Tangkanakul, W. et al. Risk factors associated with leptospirosis in northeastern Thailand, 1998. Am. J. Trop. Med. Hyg. 63, 204–208. https://doi.org/10.4269/ajtmh.2000.63.204 (2000).
Google Scholar
-
Dreyfus, A. et al. Sero-prevalence and risk factors for leptospirosis in abattoir workers in New Zealand. Int. J. Environ. Res. Public Health 11, 1756–1775. https://doi.org/10.3390/ijerph110201756 (2014).
Google Scholar
-
Della Rossa, P. et al. Environmental factors and public health policy associated with human and rodent infection by leptospirosis: A land cover-based study in Nan province, Thailand. Epidemiol. Infect. 144, 1550–1562. https://doi.org/10.1017/s0950268815002903 (2016).
Google Scholar
-
Naing, C., Reid, S. A., Aye, S. N., Htet, N. H. & Ambu, S. Risk factors for human leptospirosis following flooding: A meta-analysis of observational studies. PloS One 14, e0217643. https://doi.org/10.1371/journal.pone.0217643 (2019).
Google Scholar
-
Haake, D. A. & Levett, P. N. Leptospirosis in humans. Curr. Top. Microbiol. Immunol. 387, 65–97. https://doi.org/10.1007/978-3-662-45059-8_5 (2015).
Google Scholar
-
Vincent, A. T. et al. Revisiting the taxonomy and evolution of pathogenicity of the genus Leptospira through the prism of genomics. PLoS Negl. Trop. Dis. 13, e0007270. https://doi.org/10.1371/journal.pntd.0007270 (2019).
Google Scholar
-
Levett, P. N. Leptospirosis. Clin. Microbiol. Rev. 14, 296–326. https://doi.org/10.1128/cmr.14.2.296-326.2001 (2001).
Google Scholar
-
Zappa, A., Amendola, A., Romanò, L. & Zanetti, A. Emerging and re-emerging viruses in the era of globalisation. Blood Transfus. 7, 167–171. https://doi.org/10.2450/2009.0076-08 (2009).
Google Scholar
-
Bihl, F., Castelli, D., Marincola, F., Dodd, R. Y. & Brander, C. Transfusion-transmitted infections. J. Transl. Med. 5, 25. https://doi.org/10.1186/1479-5876-5-25 (2007).
Google Scholar
-
Singh, J. et al. Healthcare-associated hepatitis B and C transmission to patients in the EU/EEA and UK: A systematic review of reported outbreaks between 2006 and 2021. BMC Public Health 22, 2260. https://doi.org/10.1186/s12889-022-14726-0 (2022).
Google Scholar
-
Ribeiro, M. A., Cliquet, M. G. & Santos, M. G. S. Leptospirosis: A problem for transfusion medicine?. Serodiagn. Immunother. Infect. Dis. 8, 185–189. https://doi.org/10.1016/S0888-0786(96)01076-1 (1997).
Google Scholar
-
Tulsiani, S. M. et al. Emerging tropical diseases in Australia. Part 1. Leptospirosis. Ann. Trop. Med. Parasitol. 104, 543–556. https://doi.org/10.1179/136485910×12851868779867 (2010).
Google Scholar
-
Nedunchelliyan, S. & Venugopalan, A. T. Blood transfusion and leptospirosis. Indian Vet. J. 74, 790–791 (1997).
-
World Health Organization. Human Leptospirosis: Guidance for diagnosis, surveillance and control. http://www.who.int/iris/handle/10665/ 42667 (2003).
-
Limmathurotsakul, D. et al. Fool’s gold: Why imperfect reference tests are undermining the evaluation of novel diagnostics: A reevaluation of 5 diagnostic tests for leptospirosis. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 55, 322–331. https://doi.org/10.1093/cid/cis403 (2012).
Google Scholar
-
Agampodi, S. B., Dahanayaka, N. J., Nöckler, K., Mayer-Scholl, A. & Vinetz, J. M. Redefining gold standard testing for diagnosing Leptospirosis: Further evidence from a well-characterized, flood-related outbreak in Sri Lanka. Am. J. Trop. Med. Hyg. 95, 531–536. https://doi.org/10.4269/ajtmh.16-0033 (2016).
Google Scholar
-
Budihal, S. V. & Perwez, K. Leptospirosis diagnosis: Competancy of various laboratory tests. J. Clin. Diagn. Res. JCDR 8, 199–202. https://doi.org/10.7860/jcdr/2014/6593.3950 (2014).
Google Scholar
-
Jayasundara, D. et al. Optimizing the microscopic agglutination test (MAT) panel for the diagnosis of Leptospirosis in a low resource, hyper-endemic setting with varied microgeographic variation in reactivity. PLoS Negl. Trop. Dis. 15, e0009565. https://doi.org/10.1371/journal.pntd.0009565 (2021).
Google Scholar
-
Wilkinson, D. A., Edwards, M., Benschop, J. & Nisa, S. Identification of pathogenic Leptospira species and serovars in New Zealand using metabarcoding. PloS One 16, e0257971. https://doi.org/10.1371/journal.pone.0257971 (2021).
Google Scholar
-
Hinjoy, S. Epidemiology of Leptospirosis from Thai national disease surveillance system, 2003–2012. Outbreak Surveill. Investig. Rep. 7, 1–5 (2014).
-
Narkkul, U. et al. Human, animal, water source interactions and leptospirosis in Thailand. Sci. Rep. 11, 3215. https://doi.org/10.1038/s41598-021-82290-5 (2021).
Google Scholar
-
Sundharagiati, B., Harinasuta, C. & Photha, U. Human leptospirosis in Thailand. Trans. R. Soc. Trop. Med. Hyg. 60, 361–365. https://doi.org/10.1016/0035-9203(66)90300-2 (1966).
Google Scholar
-
Gonwong, S. et al. Nationwide seroprevalence of leptospirosis among young Thai Men, 2007–2008. Am. J. Trop. Med. Hyg. 97, 1682–1685. https://doi.org/10.4269/ajtmh.17-0163 (2017).
Google Scholar
-
Chadsuthi, S. et al. Investigation on predominant Leptospira serovars and its distribution in humans and livestock in Thailand, 2010–2015. PLoS Negl. Trop. Dis. 11, e0005228. https://doi.org/10.1371/journal.pntd.0005228 (2017).
Google Scholar
-
Busch, M. P. et al. Duration of dengue viremia in blood donors and relationships between donor viremia, infection incidence and clinical case reports during a large epidemic. J. Infect. Dis. 214, 49–54. https://doi.org/10.1093/infdis/jiw122 (2016).
Google Scholar
-
Lanteri, M. C. et al. West Nile virus nucleic acid persistence in whole blood months after clearance in plasma: Implication for transfusion and transplantation safety. Transfusion 54, 3232–3241. https://doi.org/10.1111/trf.12764 (2014).
Google Scholar
-
Saá, P. et al. Investigational testing for Zika virus among U.S. blood donors. N. Engl. J. Med. 378, 1778–1788. https://doi.org/10.1056/NEJMoa1714977 (2018).
Google Scholar
-
Simmons, G. et al. High incidence of Chikungunya virus and frequency of viremic blood donations during epidemic, Puerto Rico, USA, 2014. Emerg. Infect. Dis. 22, 1221–1228. https://doi.org/10.3201/eid2207.160116 (2016).
Google Scholar
-
Stone, M. et al. Zika virus RNA and IgM persistence in blood compartments and body fluids: A prospective observational study. Lancet Infect. Dis. 20, 1446–1456. https://doi.org/10.1016/s1473-3099(19)30708-x (2020).
Google Scholar
-
Stone, M. et al. Use of US blood donors for national serosurveillance of severe acute respiratory syndrome coronavirus 2 antibodies: Basis for an expanded national donor serosurveillance program. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 74, 871–881. https://doi.org/10.1093/cid/ciab537 (2022).
Google Scholar
-
Williamson, P. C. et al. First cases of Zika virus-infected US blood donors outside states with areas of active transmission. Transfusion 57, 770–778. https://doi.org/10.1111/trf.14041 (2017).
Google Scholar
-
World Health Organization. Regional Office for South-East, Asia. Regional meeting on national blood transfusion services (NBTS) to review implementation of WHO Action Framework to Advance Universal Access to Safe Blood and Blood Products. https://www.who.int/southeastasia/news/events/detail/2022/07/26/south-east-asia-events/regional-meeting-on-national-blood-transfusion-services-(nbts)-to-review-implementation-of-who-action-framework-to-advance-universal-access-to-safe-blood-and-blood-products (2022).
-
Thai Red Cross Society. Ministry of Public Health. National blood policy 2010 (Agricultural Credit Cooperatives of Thailand Publishers, 2010).
-
Faddy, H. et al. Antibodies to Leptospira among blood donors in higher-risk areas of Australia: Possible implications for transfusion safety. Blood Transfus. 13, 32–36. https://doi.org/10.2450/2014.0012-14 (2015).
Google Scholar
-
Jeevapriya, R., Pushkala, Arumugam, P. & Rajendran, A. Prevalence and detection of leptospirosis among voluntary blood donors. Int. J. Sci. Res. https://doi.org/10.36106/ijsr (2019).
Google Scholar
-
Pons, M. J. et al. Infectious agents, Leptospira spp. and Bartonella spp., in blood donors from Cajamarca, Peru. Blood Transfus. 14, 504–508. https://doi.org/10.2450/2015.0081-15 (2016).
Google Scholar
-
Jeevapriya, R. P., Arumugam, P. & Rajendran, A. Prevalence and detection of leptospirosis among voluntary blood donors. Int. J. Sci. Res. 8, 2277–8179. https://doi.org/10.36106/ijsr (2019).
Google Scholar
-
Intharasongkroh, D. et al. Hepatitis E virus infection in Thai blood donors. Transfusion 59, 1035–1043. https://doi.org/10.1111/trf.15041 (2019).
Google Scholar
-
Mohd Hanapi, I. R. et al. Prevalence of anti-Leptospira antibodies and associated risk factors in the Malaysian refugee communities. BMC Infect. Dis. 21, 1128. https://doi.org/10.1186/s12879-021-06830-0 (2021).
Google Scholar
-
Schmitz, S. et al. Risk factors for Leptospira seropositivity in rural Northern Germany, 2019. Epidemiol. Infect. 151, e17. https://doi.org/10.1017/s0950268822001972 (2022).
Google Scholar
-
Sahimin, N. et al. Seroprevalence of anti-Leptospira IgG and IgM Antibodies and risk assessment of leptospirosis among urban poor communities in Kuala Lumpur, Malaysia. Am. J. Trop. Med. Hyg. 101, 1265–1271. https://doi.org/10.4269/ajtmh.19-0003 (2019).
Google Scholar
-
Carvalho, M. D. C. et al. Serological evidence of Leptospira sp. in humans from Fernando de Noronha Island, Brazil. Comp. Immunol. Microbial. Infect. Dis. 71, 101486. https://doi.org/10.1016/j.cimid.2020.101486 (2020).
Google Scholar
-
Briskin, E. A. et al. Seroprevalence, risk factors, and rodent reservoirs of leptospirosis in an urban community of Puerto Rico, 2015. J. Infect. Dis. 220, 1489–1497. https://doi.org/10.1093/infdis/jiz339 (2019).
Google Scholar
Acknowledgements
We would like to thank all volunteers, National Blood Center in Bangkok and four regional blood centers located in Chiang Mai, Khon Kaen, Chon Buri, and Phuket province for their collaborative effort during data collection and participation in the study.
Funding
This work is supported by Tropical Medicine Cluster and Center of Excellence in Critical Care Nephrology, Faculty of Medicine, Chulalongkorn University (Nattachai Srisawat received the grant). This research project is also supported by the Second Century Fund (C2F), Chulalongkorn University (Umaporn Limothai received the grant) and Ratchadapiseksompotch Fund, Faculty of Medicine, Chulalongkorn University (Grant number GA64/05). The funders had no role in study design, data collection, and analysis, decision to publish, or manuscript preparation.
Author information
Authors and Affiliations
Contributions
S.T., J.D. and U.L. perform the ELISA, M.T. and S.B. performed the MAT, A.L. and P.W. collected data. U.L. performed data analysis, U.L. and J.S. prepared the manuscript. N.S. designed the study, supervised the research and reviewed and edited the manuscript. V.S., T.T. and U.T. supervised the research. All authors contributed to conceiving the project, interpreting results and manuscript development and review.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
Reprints and Permissions
About this article
Cite this article
Limothai, U., Tachaboon, S., Dinhuzen, J. et al. Seroprevalence of leptospirosis among blood donors in an endemic area.
Sci Rep 13, 12336 (2023). https://doi.org/10.1038/s41598-023-39461-3
-
Received: 22 March 2023
-
Accepted: 25 July 2023
-
Published: 31 July 2023
-
DOI: https://doi.org/10.1038/s41598-023-39461-3
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.