Blood
Sugar restriction and blood ingestion shape divergent immune defense trajectories in the mosquito Aedes aegypti
Abstract
Immune defense is comprised of (1) resistance: the ability to reduce pathogen load, and (2) tolerance: the ability to limit the disease severity induced by a given pathogen load. The study of tolerance in the field of animal immunity is fairly nascent in comparison to resistance. Consequently, studies which examine immune defense comprehensively (i.e. considering both resistance and tolerance in conjunction) are uncommon, despite their exigency in achieving a thorough understanding of immune defense. Furthermore, understanding tolerance in arthropod disease vectors is uniquely relevant, as tolerance is essential to the cyclical transmission of pathogens by arthropods. Here, we tested the effect(s) of dietary sucrose concentration and blood ingestion on resistance and tolerance to Escherichia coli infection in the yellow fever mosquito Aedes aegypti. Resistance and tolerance were measured concurrently and at multiple timepoints. We found that mosquitoes from the restricted sugar treatment displayed enhanced resistance at all timepoints post-infection compared to those from the laboratory standard sugar treatment. Blood also improved resistance, but only early post-infection. While sucrose restriction had no effect on tolerance, we show that consuming blood prior to bacterial infection ameliorates a temporal decline in tolerance that mosquitoes experience when provided with only sugar meals. Taken together, our findings indicate that different dietary components can have unique and sometimes temporally dynamic impacts on resistance and tolerance.
Introduction
An organism’s response to infection (i.e. immune defense) is comprised of both resistance and tolerance1,2. Resistance is defined as the ability to reduce the number of pathogens inside the body, while tolerance is defined as the ability to limit the impact of the infection on host fitness. Both strategies are critical to surviving infection. Resistance and tolerance were first conceptualized by botanists in the late 1800s3, and plant biologists have obtained major insights into both resistance and tolerance in years since. In contrast, scientists studying animal hosts have focused disproportionately on resistance until recently2,4,5. As a result, immune tolerance in animals is a nascent, but quickly developing, field of study. Tolerance strategies likely include tactics such as repair of pathogen-induced tissue damage, detoxification of pathogen by-products, limitation of immune response-mediated self-injury (i.e. immunopathology), and general homeostasis promotion during and following infection—All strategies that promote the health of the host without necessarily affecting pathogen levels2,6,7,8,9. Ecological immunology posits that resistance and tolerance, as components of host defense, are costly and should only be employed when the benefits outweigh the costs10. Therefore, a host’s balance between resistance and tolerance is likely important from a resource limitation perspective, as an inappropriate balance between resistance and tolerance may lead to an undesirable infection outcome. For example, a strong resistance response may result in complete pathogen elimination, but such an outcome could leave inadequate energy reserves for repairing infection-induced damages. If this results in reduced lifetime reproductive fitness, it would not be a successful strategy.
While an appropriate equilibrium between resistance and tolerance is important in all host–pathogen systems, it has unique relevance in hematophagous arthropods that transmit pathogens. Arthropod-borne pathogens are highly diverse, and include viruses, bacteria, protozoan parasites, and filarial worms. Diseases caused by these pathogens comprise more than 17% of all global infectious diseases11, and thus impose a massive public and veterinary health burden. Arthropod-borne pathogens are ingested by the arthropod when it takes a blood meal from an infected vertebrate host. Because transmission is categorically dependent upon the arthropod’s ability to tolerate infection long enough to spread the pathogen to a subsequent vertebrate host, studying immune tolerance is critical to the ability to understand and address arthropod-borne pathogen transmission. We therefore explored the effect of diet on resistance and tolerance to infection concurrently in the yellow fever mosquito, Ae. aegypti, which transmits multiple human pathogens including dengue virus and Zika virus.
Female adult mosquitoes have evolved to consume two meal types: (1) nectar, which is rich in sugar, and (2) blood, which is rich in protein and necessary for egg production in most species12,13. Consumption, storage, and digestion differ appreciably between the two meal types. For example, sugar meals are stored in the ventral diverticulum (crop) while blood meals bypass the crop and are directly sent to the midgut. Sugar meals can be stored in the crop until needed for energy-intensive activities such as flight while blood digestion typically begins within a few hours of feeding14. Blood digestion, unlike sucrose digestion, is also associated with significant physiological stress due to rapid shifts in temperature and pH, gut distension, heme toxicity resulting from hemoglobin digestion, and midgut redox stress15,16,17,18,19,20,21.
Diet has previously been implicated in immune defense in arthropods. For example, lower dietary sugar concentrations have been shown to enhance resistance to bacterial infection in Drosophila melanogaster and resistance to Plasmodium infection in Anopheles stephensi21,22. Effects of diet on tolerance have been explored as well. For example, genotype interacts with diet to impact tolerance to bacterial infection in D. melanogaster22. Additionally, a study that investigated the effects of a blood meal on bacterial infection in Ae. aegypti found that blood impacts resistance and tolerance early in infection, but the effects were dose-dependent23. In the present study, we tested the effects of two adult female Ae. aegypti diet components, (1) lab standard or restricted dietary sucrose, and (2) the ingestion of a blood meal, on resistance and tolerance to bacterial infection. We measured resistance and tolerance concurrently at multiple timepoints across a 5-day infection timecourse, allowing us to examine each treatment group’s resistance/tolerance trajectory, as well as test for any potential interactions between the two components of the mosquito’s diet in affecting immune defense. Our results contribute to the understanding of how arthropods of medical importance withstand bacterial infection, and more specifically, elucidate effects of blood feeding and sugar feeding on immune defense that may be conserved amongst other hematophagous arthropod vectors.
Results
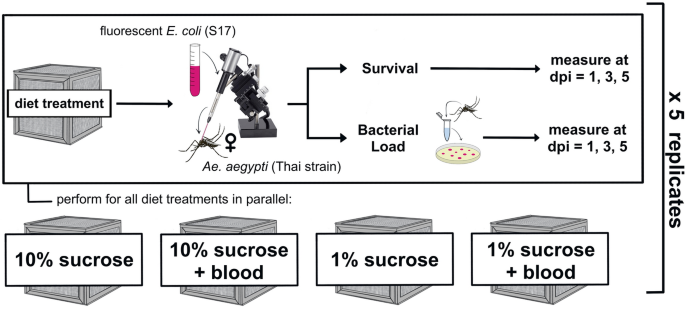
We investigated the effect of dietary sucrose concentration and blood feeding on resistance and tolerance to infection over time. To accomplish this, we exposed female Ae. aegypti mosquitoes to four different diet treatments: 10% sucrose alone, 10% sucrose + blood meal, 1% sucrose alone, and 1% sucrose + blood meal. We then infected them with E. coli (S17) and measured bacterial load and survival at multiple timepoints post-infection (Fig. 1). The resultant dataset comprises seven variables (Table 1) and was used to build multiple models describing the effect(s) of both Blood and Sucrose on resistance and tolerance to bacterial infection across time.
Experimental design schematic. We reared adult female Ae. aegypti on four experimental diets: 10% sucrose alone, 10% sucrose + blood meal, 1% sucrose alone, and 1% sucrose + blood meal. We then infected individuals with fluorescent E. coli (S17) via intrathoracic microinjection. Infected mosquitoes were split into monitoring groups for survival and bacterial load. Survival and bacterial load measurements were obtained in parallel at 1, 3, and 5 days post-infection (dpi). The resultant dataset was used to build models describing the effect(s) of blood and/or sucrose diets on resistance and tolerance simultaneously (Table 1).
Dietary sucrose restriction and blood ingestion enhance resistance
We measured resistance to bacterial infection by testing the effects of Sucrose and Blood on Bacterial Load at 1, 3, and 5 days post-infection (dpi). We first built a model using Sucrose, Blood, and Day as predictor variables and Bacterial Load as the response variable, then assessed main effects as well as all potential interactions. We performed backward elimination to identify significant model terms and found that both Sucrose (Table 2: Model A, p Sucrose = 4.57 × 10–5) and Day (Table 2: Model A, p Day = 2.92 × 10–4) significantly affect resistance to infection. Females fed 1% sucrose had significantly lower bacterial loads (and therefore higher resistance) than females fed 10% sucrose, and bacterial load decreased over time for both treatment groups (Fig. 2). Furthermore, pairwise comparisons between sucrose treatments on each individual day showed that 1% sucrose exposure significantly enhanced resistance to infection at all days post-infection. (Table 3: Model B (Day 1), p Sucrose = 0.008; Model C (Day 3), p Sucrose = 0.005; Model D (Day 5), p Sucrose = 0.048).

Mosquitoes fed 1% sucrose had significantly lower bacterial loads compared to mosquitoes fed 10% sucrose. Boxplots were constructed using median bacterial loads for females fed 1% sucrose and 10% sucrose at 1, 3, and 5 dpi. Each median value was calculated from four individuals, and point error bars show the interquartile range. Asterisks represent significant differences in bacterial load between sucrose treatments at each time post-infection (**p < 0.01, *p < 0.05). Data were collected from a total of five replicate experiments.
Blood feeding also significantly affected resistance to infection, but only at 1 dpi, at which time blood-fed females had significantly lower bacterial loads compared to non-blood-fed females (Table 3: Model B, p Blood = 0.038; Fig. 3).

Blood-fed mosquitoes had significantly lower bacterial loads compared to non-blood-fed mosquitoes at 1 dpi. Boxplots were constructed using plotted points showing median (n = 4) bacterial load for blood-fed and non-blood-fed females at 1, 3, and 5 dpi. Each median value was calculated from four individuals, and point error bars show the interquartile range. Asterisks represent significant differences in bacterial load between sucrose treatments at each time post-infection (*p < 0.05, ns = no significant difference). Data were collected from a total of five replicate experiments.
The effect of a blood meal on tolerance is dynamic across infection timecourse
We also tested the effects of Blood and Sucrose on tolerance to infection, where tolerance was measured as the slope of population survival regressed on pathogen load24. To compare tolerance between treatment groups, we determined the presence or absence of a statistically significant interaction term between Bacterial Load and a treatment variable in predicting Survival in a given model. The presence of such a significant interaction term would indicate that the relationship between Survival and Bacterial Load changes depending on the value of the treatment variable.
We first built a model with Survival as the response variable and Bacterial Load, Blood, Sucrose, and Day as the predictor variables. We assessed main effects as well as all potential interactions and performed backward elimination to achieve the best fit model. The final model revealed a significant three-way interaction between Blood, Bacterial Load, and Day (Table 4: Model E; p Bacterial Load × Blood × Day = 0.006), indicating that: (1) blood significantly alters tolerance, and (2) the effect changes across time. In order to more closely examine the nature of blood’s time-dependent effect on tolerance, we parsed our data by day and used the resulting three datasets to build a tolerance model for each day (Fig. 4). These day-specific models reveal that blood-fed mosquitoes had significantly lower tolerance at 1 dpi (Table 5: Model F, p Bacterial Load × Blood = 0.002), no significant difference at 3 dpi, and significantly higher tolerance at 5 dpi (Table 5: Model G, p Bacterial Load × Blood = 0.040) when compared to non-blood-fed mosquitoes. In parallel, we also tested whether tolerance changes across time for blood-fed and/or non-blood-fed treatment groups. To achieve this, we parsed our dataset by blood feeding status and used the resulting two datasets to build a tolerance model for each blood feeding treatment group. These models revealed that tolerance significantly decreased over time in non-blood-fed mosquitoes (Table 6: Model H, p Bacterial Load × Day = 6.70 × 10–6) but did not significantly change in blood-fed mosquitoes (Table 6: Model I).

The effect of blood feeding on tolerance varies across time. Points show population survival plotted against median bacterial load at each timepoint post-infection. Error bars around points show the interquartile range of the four values used to calculate bacterial load median values. Plotted lines are derived from interaction plots of estimated marginal means calculated from day-specific binomial tolerance models (Table 5: Model F (Day 1), pBacterial Load × Blood = 0.002; Day 3, no significant predictors; Table 5: Model G (Day 5), pBacterial Load × Blood = 0.040) plotted on a response scale rather than a linear scale. The presence of solid lines indicates significantly different slopes, and therefore a significant difference in tolerance, between blood treatment groups at that timepoint. Dashed lines indicate no significant difference in tolerance at that timepoint. Data were collected from a total of five replicate experiments.
A blood meal alters the shape of a mosquito’s immune defense trajectory through time
Blood altered resistance early in infection and tolerance dynamically across the infection timecourse. Therefore, we compared the relative utilization of each component of immune defense by quantitatively plotting resistance and tolerance for each blood feeding status group at all three timepoints (Fig. 5). Blood-fed mosquitoes displayed an increase in resistance (Table 2: Model A, p Day = 2.92 × 10–4) and a non-significant trend toward increased tolerance (Table 6: Model I) from 1 to 5 dpi, while non-blood-fed mosquitoes displayed increased resistance (Table 2: Model A, p Day = 2.92 × 10–4) but decreased tolerance (Table 6: Model H, p Bacterial Load × Day = 6.70 × 10–6) from 1 to 5 dpi (Fig. 5).

Blood feeding alters the mosquito’s trajectory of resistance and tolerance over time. Plotted points display the balance of tolerance and resistance through an infection timecourse for blood-fed (left panel) and non-blood-fed mosquitoes (right panel). To estimate tolerance, we regressed Bacterial load on Survival separately for each blood feeding status/day combination and found the estimated marginal mean and standard error of Bacterial Load for each model using the emmeans package111. Resistance is the population median CFU with interquartile ranges for each group plotted on a reversed x-axis scale (as resistance is inversely related to CFU). Data were collected from a total of five replicate experiments.
Discussion
Restricting sucrose enhances resistance throughout infection timecourse
We have shown that a 1% sucrose diet confers higher resistance to intrathoracic infection by the gram-negative bacterium E. coli S17 in adult female Ae. aegypti (Thai strain) compared to a 10% sucrose diet. In mosquitoes, effects of dietary sugars on infection response have primarily been studied using oral infection models. In one study, pre-infection sugar feeding enhanced Ae. aegypti resistance to Zika by increasing the expression of antiviral genes25, while in An. stephensi, lower dietary glucose abundance correlated with higher resistance to Plasmodium berghei21. In D. melanogaster, lower dietary sugar concentration increased resistance to bacterial infection in two separate studies22,26. Altogether, the current body of work suggests lower dietary sugar levels usually correlate with increased resistance to infection, which is consistent with our findings.
While dietary sucrose significantly affected mosquito resistance in our study, it did not affect survival (S1 Fig). Conversely, effects of dietary sugar on survival have been observed in D. melanogaster, although the directionality of the effect is not consistent. Howick & Lazzaro22 showed that higher dietary sugars were associated with lower survival (and lower resistance) post-infection. However, other studies have shown a positive relationship between dietary sugar and infection outcome (although resistance was not directly measured). For example, in one study, flies that consumed a relatively low-protein, high-carbohydrate diet displayed increased survival to bacterial infection and higher constitutive expression of antimicrobial peptides (AMPs)27. In another study, dietary glucose supplementation led to significantly improved D. melanogaster longevity and survival following bacterial infection28. Multiple dissimilarities between these studies and our own, including experimental design and pathogen type, could underlie the different findings. Overall, relationships between dietary sugars and survival after infection are not fully consistent and warrant further study.
The mechanisms by which sugar affects resistance are not known. As diet is known to affect hemolymph composition in insects29,30, higher dietary sugar may provide a more suitable environment for bacteria to proliferate. Alternatively, but not mutually exclusively, a lower concentration of dietary sucrose may directly or indirectly affect signaling of the mosquito’s immune pathways, thereby inhibiting resistance mechanisms such as melanization, AMP production, and/or hemocyte activity. Higher dietary sugar levels are associated with a stronger melanization response in mosquitoes31,32,33, which may initially appear antithetical to our results. However, E. coli is preferentially phagocytized rather than melanized by mosquitoes34,35,36, suggesting that any reduction in melanization that may have occurred in the 1% sucrose treatment is unlikely to impact infection outcomes in our system. Sucrose may have a different effect after challenge with a pathogen that is primarily melanized. In D. melanogaster, differences in dietary sugar levels are associated with physiological changes that may explain effects on resistance. For example, flies reared on high sugar diets display abnormal hemocyte morphology, defects in phagocytosis ability, and excessive activation of Toll/JNK in the fat body37. Other work has also implicated high-sugar diets in inhibition of FOXO signaling in D. melanogaster38, which has been shown to be critical in resistance to bacterial infection39.
The sucrose diets provided to mosquitoes in this laboratory setting are not representative of sugar meals obtained in the field. Mosquito sugar feeding behavior in natural settings is not fully understood; however, evidence suggests that mosquitoes can obtain sugar in natural settings by feeding on floral nectar, extrafloral nectar, honeydew, tree sap, rotting fruit, sugar cane trash, and even sugar-rich household waste12,14. The compositions of the sugar meals consumed by mosquitoes in natural settings are quite variable. For example, floral nectar meals typically include a variety of different sugars, while household waste items may contain non-naturally occurring substances such as high-fructose corn syrup or fruit juice concentrates. In the present study, we have explored two concentrations of one type of sugar in isolation. Further study is warranted to determine the effects of sugar concentrations or different types of sugar on resistance and tolerance to infection.
While the differences in immune resistance between our sucrose treatments may be explained by the effect of dietary sucrose availability per se, it is also feasible that the effect we observe is the result of general caloric restriction. Insects subject to food restriction and insects subject to infection undergo many of the same physiological changes, including reconfiguration of intermediate metabolism, reduced energy storage, release of glucose and fatty acids from existing energy stores, and inhibition of the insulin-like signaling pathway40,41. By virtue of this commonality, food restriction prior to infection may induce a metabolic switch that functionally primes the mosquito to resist pathogen invasion. Indeed, positive effects of starvation or dietary restriction on resistance and/or infection outcome more generally have been observed in multiple insect orders42,43,44,45,46,47. Imposed starvation does not always lead to increased resistance, however, and in many cases has been shown to cause decreased resistance and/or adverse infection outcomes44,48,49,50,51,52. For example, starved Leptinotarsa decemlineata beetles display increased susceptibility to Beauveria bassiana and heightened mortality post-infection compared to their non-starved counterparts49. Similarly, the tsetse fly Glossina morsitans morsitans displays increased susceptibility to both Trypanosoma congolense or Trypanosoma brucei brucei under starvation conditions50. The effects of starvation on response to infection are not uniform across taxa. These differences may be explained by a variety of factors such as pathogen type, pathogen dose, infection route, or host life history idiosyncrasies, but further studies are needed to understand the relationship between starvation and immune defense.
Blood improves resistance early post-infection
Our data also reveal that a blood meal enhances resistance at 1 dpi. This differs from the effect of sucrose, which remains significant at every timepoint measured. Previous work reported similar effects of blood feeding on resistance to E. coli, in association with insulin signaling in one instance23 and 20-hydroxyecdysone (20E) in another53. In addition to supporting these findings, our data contribute an enhanced understanding of the dynamic nature of blood’s effect over time. Specifically, the effect of blood on resistance is relatively transient, especially in comparison to the effect of dietary sucrose on bacterial resistance which is consistent for at least five days post-infection. As the effect of blood on 20E titers generally does not extend past 48 h post-blood meal54,55 and 20E can enhance resistance in mosquitoes (Reynolds et al.53; but see Wang et al.56; Werling et al.57), it is possible that the dynamic effect of blood feeding on resistance we observed is due to changing 20E titers over time. Titers of another hormone, Juvenile Hormone (JH), rapidly and drastically decline in blood-fed Ae. aegypti, reaching minimums between 24 and 48 h post-blood meal58,59. JH and JH analogs are associated with various immunosuppressive effects, including the downregulation of AMP genes60,61,62, decreased activity of hemocyte activator molecules63, and reductions in phenoloxidase activity64,65. Therefore, it is also feasible that improved resistance after blood feeding is mediated via declines in JH. In addition to the potential regulatory role of hormones, investigations into the mosquito’s transcriptional profile post-blood feeding indicate altered expression of immune-related genes within the 24 h following a blood meal66,67,68,69. Such genes may affect resistance to bacterial infection and may or may not be subject to regulation by insulin signaling and/or hormones such as JH and 20E.
Blood ingestion, but not sucrose restriction, alters tolerance to infection
While the effect of blood on resistance is limited to 1 dpi, we observe a dynamic effect of blood feeding on tolerance across the 5-day infection timecourse. Blood-fed mosquitoes have significantly lower tolerance compared to non-blood-fed mosquitoes at 1 dpi, and significantly higher tolerance at 5 dpi. Further, non-blood-fed mosquitoes show a significant decrease in tolerance across a 5-day infection timecourse while blood-fed mosquitoes show static tolerance across a 5-day infection timecourse. This suggests that the blood meal ameliorates a decline in tolerance that mosquitoes experience when provided only sugar meals. Blood is a unique nutritional resource – its digestion is associated with various physiological stressors, including rapid shifts in temperature and pH15,70 as well as heme toxicity and oxidative stress71,72,73. Despite the challenges posed by blood digestion, mosquitoes are well adapted to overcome the associated stressors15,17,70,74,75. Because many stressors associated with blood feeding are also associated with infection20,21,76,77 it is possible that the homeostasis-promoting processes that occur during or after blood feeding have the additional effect of promoting host health during infection (i.e. tolerance).
Blood feeding induces multiple signaling cascades that may promote tolerance to infection. For example, heat shock proteins (HSP) are widely conserved in both eukaryotes and prokaryotes78 and are implicated in a variety of processes generally related to the reduction of stress79. They are rapidly induced upon blood feeding in multiple arthropods and are critical to surviving the stress of a blood meal15,19,80. Host HSPs have also been implicated in host response to bacterial, viral, and fungal infections alike, playing roles in homeostasis via maintenance of protein stability and functionality, reductions in inflammation, and attenuation of autoimmunity81,82. HSPs that protect arthropods from blood-induced damage may also promote host health and survival during infection. Additionally, the unfolded protein response (UPR) is a similarly highly conserved, stress-mitigating, pathway that has been heavily implicated in defense against pathogens (reviewed by Rosche et al.83). Transient UPR upregulation occurs after blood feeding in Ae. aegypti84. Further, UPR upregulation promotes tolerance to Pseudomonas aeruginosa in the nematode Caenorhabditis elegans85. It also promotes survival in mosquito cells infected with dengue virus in vitro by ameliorating infection-induced endoplasmic reticulum stress86, a function which is characteristic of tolerance. Importantly, it is unlikely that any single signaling pathway or molecular cascade regulates tolerance to infection, as immunopathology affects various physiological processes1 that require repair or protection. Thus, if the aforementioned processes do indeed affect tolerance, they likely do so in tandem with other mechanisms.
It is also possible that the tolerance benefit of a blood meal is explained by the influx of nutrients (e.g. protein, lipid) associated with a blood meal. However, we observed no effect of sucrose on tolerance, and higher dietary sucrose concentrations are also strongly associated with higher nutrient reserves. Whole-body homogenates of mosquitoes fed 10% sucrose have significantly higher levels of sugar, glycogen, and lipids compared to mosquitoes fed 2% sucrose87. This indicates that if tolerance is indeed regulated nutritionally in our system, this regulation may be specific to the nutrients provided by a blood meal.
Blood-fed and non-blood-fed mosquitoes employ different, but equally effective, immune defense strategies
Blood-fed and non-blood-fed mosquitoes showed markedly different resistance-tolerance strategies for surviving infection with a non-coevolved bacterium (Fig. 5). In the absence of a blood meal, a mosquito displays an increase in resistance and a decrease in tolerance across a 5-day infection timecourse. But when provided with a blood meal, early infection is characterized by a significantly stronger resistance response and significantly weaker tolerance response (possibly the result of a trade-off). Across time, blood-fed mosquitoes show no change in tolerance, and an increase in resistance that is similar to that of non-blood-fed mosquitoes. In comparing the groups at the end of infection timecourse, blood-fed mosquitoes show equal resistance and higher tolerance compared to non-blood-fed mosquitoes. Each diet clearly induced a unique immune defense strategy. Interestingly, we observed no significant difference in survival (S1 Fig) indicating that the two strategies employed by the groups are equally effective in surviving infection. We detected no statistically significant interactions between Sucrose and Blood in our models, indicating that the effects of blood on resistance, tolerance, and survival did not differ between 1% sucrose and 10% sucrose groups. However, it is possible that alternative sucrose regimens (e.g. a sucrose concentration higher than 10% or the complete absence of any sugar meal) not tested herein may indeed alter the effect of blood.
A blood meal is not only physiologically stressful and energetically costly to digest on its own88, it also catalyzes reproductive processes (i.e. vitellogenesis) that are energetically costly89. Immune defense is also a costly process10,90,91,92. Understanding how organisms partition limited resources amongst energy-intensive processes represents an ongoing challenge in biology. Reproduction, immunity, and digestion have been shown to physiologically trade off in multiple insect systems89,93,94,95,96,97,98,99. In light of this, it may be somewhat surprising that mosquitoes under the combined stress of blood digestion, reproduction, and infection in parallel show no difference in survival compared to non-blood-fed mosquitoes. Overall, our results therefore suggest that the immune defense benefits of a blood meal are great enough to mitigate the stressors and resource usage associated with this meal.
Conclusions
In the present study, we show the effects of blood ingestion and two dietary sucrose concentrations on both resistance and tolerance to the non-coevolved bacterium E. coli in the adult female yellow fever mosquito Ae. aegypti. Our results indicate that dietary sucrose concentration and blood ingestion both affect resistance, while only blood ingestion affects tolerance. The effect of blood on tolerance was dynamic across time, significantly worsening tolerance at the start of the infection timecourse and significantly enhancing tolerance by the end. Mosquitoes are one of many arthropods that transmit pathogens by virtue of a hematophagous lifestyle: mosquitoes, ticks, biting midges, triatomine bugs, fleas, black flies, and sand flies alike all transmit pathogens when consuming vertebrate blood100. Likewise, they all share the ability to tolerate the pathogens they transmit, which is critical for successful transmission. In light of this, motivation for exploring the relationship between hematophagy and tolerance is not limited to mosquitoes, but rather, is broadly relevant in all arthropod-borne pathogen systems. Our study focused on bacterial infection introduced by septic wound; additional work to investigate the role of diet in tolerance to oral or vertical pathogen infection is also needed and would provide critical insight into the generalizability of our findings. Shared characteristics of tolerance biology across arthropod species, modes of infection, or pathogen type could potentially be leveraged as novel targets for effective and sustainable vector control101. Because blood feeding is already one of the best-described areas of mosquito biology, has been investigated102,103,104,105 and implemented106 as a vector control target, and affects tolerance in Ae. aegypti, targeting blood feeding in the context of tolerance-focused vector control holds excellent potential.
Methods
Mosquitoes
Throughout the duration of the experiment, Ae. aegypti Thai strain (Laura C. Harrington, Cornell University) mosquitoes were reared in a chamber maintained at 27 °C and 80% relative humidity under a 14 h:10 h light:dark cycle. First, eggs were hatched in RO (reverse osmosis) water placed in a vacuum chamber. Upon hatching, larvae were reared in trays containing RO water at a density of 200–300 larvae per tray and given one pinch of Tetramin fish flakes as well as cat food ad libitum until pupation. Upon pupation, pupal cups were split into four 8″ × 8″ mesh treatment cages (Bioquip, Rancho Dominguez, CA, USA): 10% sucrose only, 10% sucrose + blood, 1% sucrose only, and 1% sucrose + blood. Each cage received the appropriate sucrose meal concurrent with the addition of pupal cups. Pupae were allotted 48 h to eclose before pupal cup removal. Post-eclosion, all adults were left undisturbed for 48 h, then starved for 24 h. Next, mosquitoes from cages containing blood feeding treatments were provided with a blood meal maintained at 37 °C via a membrane feeding system (Hemotek, Blackburn, UK) for 1–2 h. Sucrose meals were then returned to all mosquitoes. Infections were performed on females at 24 h post-blood feeding. At this time, blood feeding status was confirmed visually under a microscope by the presence of a blood bolus.
Sucrose and blood diets
Mosquitoes were maintained on one of four experimental diets: 10% sucrose alone, 10% sucrose + blood, 1% sucrose alone, and 1% sucrose + blood. Sucrose meals were created by passing a solution of DI (deionized) water and UltraPure sucrose (Life Technologies, Carlsbad, CA) through a 0.2 µm filter. Blood meals consisted of defibrinated rabbit blood (Hemostat, Dixon, CA, USA) supplemented with Na2ATP to a 1 mM concentration.
Bacterial culture and mosquito infections
The bacteria used for infections, E. coli S17 pPROBE-mCherry (Dimopoulos Lab, unpublished data), contains a fluorescent mCherry plasmid and a kanamycin resistance cassette. Bacteria were grown in Luria broth (LB) supplemented with kanamycin (50 µg/mL) overnight at 30 °C with shaking. Cultures were washed thrice in sterile 1X PBS, then pelleted and resuspended to OD600 = 1 ± 0.1 (mean of 1.23 × 9 CFU/mL across replicates). At the time of infection, mosquitoes were anesthetized on ice and females were injected with 69 nanoliters of a 1 × 10–2 dilution of this culture by piercing the soft tissue of the anepisternal cleft of the mesothorax using a Nanoject II Auto-Nanoliter Injector (Drummond, Broomall, PA, USA). Fresh injection needles were prepared for each replicate by manually pulling a borosilicate glass capillary tube (Drummond, Broomall, PA, USA) over a flame to achieve a tip with an outer diameter no greater than 500 microns as measured using a stage micrometer. Preliminary experiments indicated that Nanoject II delivery of bacteria was adequately precise, delivering a mean of 312 ± 13 CFU per mosquito (n = 6 mosquitoes were injected, then each homogenate was immediately cultured as described in the subsequent section) (S1 File). Following infection, mosquitoes from each diet group were randomly allocated into two groups and placed in separate cages for survival and bacterial load measurements.
Monitoring survival and bacterial load
Survival and bacterial load were measured on days 1 (24 h + /– 2 h), 3 (72 h + /– 2 h), and 5 (120 h + /– 2 h) post-infection. Immediately after data collection at each timepoint, dead individuals were removed from survival and bacterial load cages and discarded. Survival was monitored by counting the number of mosquitoes dead and alive. To measure bacterial load, four living mosquitoes were sampled using an InsectaVac Aspirator (Bioquip, Rancho Dominguez, CA, USA) and individually homogenized in 150 µL sterile 1X PBS. Serial dilutions were performed at 10–2 and 10–4 and cultured alongside undiluted homogenate on LB supplemented with kanamycin (50 µg/mL) at 30 °C for 24–48 h. Resulting fluorescent colonies were counted using a stereo microscope fluorescence adapter system (Nightsea, Lexington, MA). For each individual, the least dilute plate with countable CFUs was used to obtain a representative value. After obtaining a CFU count for each individual mosquito, the median of four mosquitoes was calculated and used in the data set. Each row in the data set (S1 Dataset) contains the median CFU of four mosquitoes paired with the accompanying survival value for that treatment group.
Statistical analysis
All analyses were performed using R statistical software107 and RStudio108.
To measure resistance, we built linear models testing the effect(s) of Blood, Sucrose, Day, and Replicate on Bacterial Load (Table 1). Backward elimination was used to assess all possible interactions as well as main effects. The presence of any main effect indicates that variable significantly affects resistance.
To measure tolerance, we used a reaction norm approach24 to test for variation in health across a range of real-time bacterial loads between diet groups at multiple time points. Our approach is thus categorized as range tolerance rather than point tolerance109. We built binomial generalized linear models testing the effect(s) of Blood, Sucrose, Day, and Bacterial Load on Survival (Table 1). Backward elimination was used to assess all possible interactions as well as main effects. Further, we tested all tolerance models for overdispersion110 by performing residual deviance goodness-of-fit tests and Pearson goodness-of-fit tests. When detected, we adjusted for overdispersion by correcting the standard errors in those models through incorporation of a dispersion parameter when calculating the variance. Models with overdispersion parameters are defined as quasi-generalized linear models using a binomial distribution where the variance is given by φ × μ, in which μ is the mean and φ the dispersion parameter. We used these final models to interpret any effect(s) of our predictor variables on tolerance. Specifically, a significant interaction between Bacterial Load and another variable in predicting Survival indicates that variable significantly affects tolerance.
To obtain interaction plots of estimated marginal means for each day-specific binomial tolerance model (Fig. 4), we used the emmip function from the emmeans package111. To obtain representative values for tolerance for each level of Blood on Days 1, 3, and 5 (Fig. 5), we used the emtrends function from the emmeans package, a function that creates a reference grid for each model of interest, then calculates difference quotients of predictions from those reference grids and computes the marginal averages and standard errors for those averages. We used the emtrends function to obtain these values for each day using Model F (Day 1), Model G (Day 5), and a model with a non-significant Bacterial Load × Blood term for Day 3 (model not shown).
Data availability
All data generated or analyzed during this study are included in this published article (and its Supplementary Information files).
References
-
Medzhitov, R., Schneider, D. S. & Soares, M. P. Disease tolerance as a defense strategy. Science 335, 936–941 (2012).
Google Scholar
-
Schneider, D. S. & Ayres, J. S. Two ways to survive infection: What resistance and tolerance can teach us about treating infectious diseases. Nat. Rev. Immunol. 8, 889–895 (2008).
Google Scholar
-
Cobb, N. A. Contributions to an Economic Knowledge of Australian Rusts (Uredineae). [Chapter X]. Improving Wheat by Selection (Department of Agriculture, 1894).
-
Corby-Harris, V., Habel, K. E., Ali, F. G. & Promislow, D. E. L. Alternative measures of response to Pseudomonas aeruginosa infection in Drosophila melanogaster. J. Evol. Biol. 20, 526–533 (2007).
Google Scholar
-
Råberg, L., Sim, D. & Read, A. F. Disentangling genetic variation for resistance and tolerance to infectious diseases in animals. Science 318, 812–814 (2007).
Google Scholar
-
McCarville, J. L. & Ayres, J. S. Disease tolerance: Concept and mechanisms. Curr. Opin. Immunol. 50, 88–93 (2018).
Google Scholar
-
Råberg, L., Graham, A. L. & Read, A. F. Decomposing health: Tolerance and resistance to parasites in animals. Philos. Trans. R. Soc. B Biol. Sci. 364, 37–49 (2009).
Google Scholar
-
Read, A. F., Graham, A. L. & Råberg, L. Animal defenses against infectious agents: Is damage control more important than pathogen control. PLoS Biol. 6, e1000004 (2008).
Google Scholar
-
Soares, M. P., Teixeira, L. & Moita, L. F. Disease tolerance and immunity in host protection against infection. Nat. Rev. Immunol. 17, 83–96 (2017).
Google Scholar
-
Baucom, R. S. & de Roode, J. C. Ecological immunology and tolerance in plants and animals. Funct. Ecol. 25, 18–28 (2011).
Google Scholar
-
World Health Organization. Vector-Borne Diseases (World Health Organization, 2022).
-
Barredo, E. & DeGennaro, M. Not just from blood: Mosquito nutrient acquisition from nectar sources. Trends Parasitol. 36, 473–484 (2020).
Google Scholar
-
Wolff, G. H. & Riffell, J. A. Olfaction, experience and neural mechanisms underlying mosquito host preference. J. Exp. Biol. 221, jeb157131 (2018).
Google Scholar
-
Foster, W. A. Mosquito sugar feeding and reproductive energetics. Annu. Rev. Entomol. 40, 443–474 (1995).
Google Scholar
-
Benoit, J. B. et al. Drinking a hot blood meal elicits a protective heat shock response in mosquitoes. Proc. Natl. Acad. Sci. 108, 8026–8029 (2011).
Google Scholar
-
Champion, C. J. & Xu, J. The impact of metagenomic interplay on the mosquito redox homeostasis. Free Radic. Biol. Med. 105, 79–85 (2017).
Google Scholar
-
Lahondère, C. & Lazzari, C. R. Mosquitoes cool down during blood feeding to avoid overheating. Curr. Biol. 22, 40–45 (2012).
Google Scholar
-
Oliveira, M. F. et al. Haem detoxification by an insect. Nature 400, 517–518 (1999).
Google Scholar
-
Pereira, M. H., Paim, R. M. M., Lahondère, C. & Lazzari, C. R. Heat Shock Proteins in Veterinary Medicine and Sciences Heat Shock Proteins and Blood-Feeding in Arthropods, 349–359 (Springer International Publishing, 2017).
Google Scholar
-
Peterson, T. M. L. & Luckhart, S. A mosquito 2-Cys peroxiredoxin protects against nitrosative and oxidative stresses associated with malaria parasite infection. Free Radic. Biol. Med. 40, 1067–1082 (2006).
Google Scholar
-
Wang, M. et al. Glucose-mediated proliferation of a gut commensal bacterium promotes Plasmodium infection by increasing mosquito midgut pH. Cell Rep. 35, 108992 (2021).
Google Scholar
-
Howick, V. M. & Lazzaro, B. P. Genotype and diet shape resistance and tolerance across distinct phases of bacterial infection. BMC Evol. Biol. 14, 56 (2014).
Google Scholar
-
Castillo, J., Brown, M. R. & Strand, M. R. Blood feeding and insulin-like peptide 3 stimulate proliferation of hemocytes in the mosquito Aedes aegypti. PLoS Pathog. 7, e1002274 (2011).
Google Scholar
-
Simms, E. L. Defining tolerance as a norm of reaction. Evol. Ecol. 14, 563–570 (2000).
Google Scholar
-
Almire, F. et al. Sugar feeding protects against arboviral infection by enhancing gut immunity in the mosquito vector Aedes aegypti. PLoS Pathog. 17, e1009870 (2021).
Google Scholar
-
Unckless, R. L., Rottschaefer, S. M. & Lazzaro, B. P. The complex contributions of genetics and nutrition to immunity in Drosophila melanogaster. PLoS Genet. 11, e1005030 (2015).
Google Scholar
-
Ponton, F. et al. Macronutrients modulate survival to infection and immunity in Drosophila. J. Anim. Ecol. 89, 460–470 (2020).
Google Scholar
-
Galenza, A., Hutchinson, J., Campbell, S. D., Hazes, B. & Foley, E. Glucose modulates Drosophila longevity and immunity independent of the microbiota. Biol. Open 5, 165–173 (2016).
Google Scholar
-
Mullins, D. E. Compr. Insect Physiol., Vol. 3,Chemistry and Physiology of the Hemolymph, 355–400 (1985).
-
Thompson, S. N. Advances in Insect Physiology Vol. 31, Trehalose-The Insect ‘Blood Sugar,’ 205–285 (Elsevier, 2003).
-
Ferguson, L. V. et al. Sugar intake interacts with temperature to influence reproduction and immunity in adult Culex pipiens mosquitoes. Can. J. Zool. 97, 424–428 (2019).
Google Scholar
-
Koella, J. C. & Sørensen, F. L. Effect of adult nutrition on the melanization immune response of the malaria vector Anopheles stephensi. Med. Vet. Entomol. 16, 316–320 (2002).
Google Scholar
-
Schwartz, A. & Koella, J. C. Melanization of Plasmodium falciparum and C-25 sephadex beads by field-caught Anopheles gambiae (Diptera: Culicidae) from Southern Tanzania. J. Med. Entomol. 39, 84–88 (2002).
Google Scholar
-
Hernández-Martínez, S., Lanz, H., Rodrguez, M. H., González-Ceron, L. & Tsutsumi, V. Cellular-mediated reactions to foreign organisms inoculated into the hemocoel of Anopheles albimanus (Diptera: Culicidae). J. Med. Entomol. 39, 61–69 (2002).
Google Scholar
-
Hillyer, J. F., Schmidt, S. L. & Christensen, B. M. Hemocyte-mediated phagocytosis and melanization in the mosquito Armigeres subalbatus following immune challenge by bacteria. Cell Tissue Res. 313, 117–127 (2003).
Google Scholar
-
Hillyer, J. F., Schmidt, S. L. & Christensen, B. M. Rapid phagocytosis and melanization of bacteria and Plasmodium sporozoites by hemocytes of the mosquito Aedes aegypti. J. Parasitol. 89, 62–69 (2003).
Google Scholar
-
Yu, S., Zhang, G. & Jin, L. H. A high-sugar diet affects cellular and humoral immune responses in Drosophila. Exp. Cell Res. 368, 215–224 (2018).
Google Scholar
-
Dobson, A. J. et al. Nutritional programming of lifespan by FOXO inhibition on sugar-rich diets. Cell Rep. 18, 299–306 (2017).
Google Scholar
-
Fink, C. et al. Intestinal FoxO signaling is required to survive oral infection in Drosophila. Mucosal Immunol. 9, 927–936 (2016).
Google Scholar
-
Adamo, S. A. How insects protect themselves against combined starvation and pathogen challenges, and the implications for reductionism. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 255, 110564 (2021).
Google Scholar
-
Adamo, S. A. & Miyashita, A. Adv. Invertebr. Neuroendocrinol. Collect. Rev. Post-Genomic Era Vol. 2 Arthropoda 2, Stayin’ alive: Endocrinological Stress Responses in Insects, 283–323 (2020).
-
Brown, A. E., Baumbach, J., Cook, P. E. & Ligoxygakis, P. Short-term starvation of immune deficient Drosophila improves survival to gram-negative bacterial infections. PLoS One 4, e4490 (2009).
Google Scholar
-
Burger, J. M. S., Hwangbo, D. S., Corby-Harris, V. & Promislow, D. E. L. The functional costs and benefits of dietary restriction in Drosophila. Aging Cell 6, 63–71 (2007).
Google Scholar
-
Conroy, T. J., Palmer-Young, E. C., Irwin, R. E. & Adler, L. S. Food limitation affects parasite load and survival of Bombus impatiens (Hymenoptera: Apidae) infected with Crithidia (Trypanosomatida: Trypanosomatidae). Environ. Entomol. 45, 1212–1219 (2016).
Google Scholar
-
Kang, K.-D., Kamita, S. G., Suzuki, K. & Seong, S.-I. Effect of starvation upon baculovirus replication in larval Bombyx mori and Heliothis virescens. J. Invertebr. Pathol. 106, 205–210 (2011).
Google Scholar
-
Logan, A., Ruiz-González, M. X. & Brown, M. J. F. The impact of host starvation on parasite development and population dynamics in an intestinal trypanosome parasite of bumble bees. Parasitology 130, 637–642 (2005).
Google Scholar
-
Moller-Jacobs, L. L., Murdock, C. C. & Thomas, M. B. Capacity of mosquitoes to transmit malaria depends on larval environment. Parasit. Vectors 7, 593 (2014).
Google Scholar
-
Akoda, K. et al. Maturation of a Trypanosoma Brucei infection to the infectious metacyclic stage is enhanced in nutritionally stressed Tsetse flies. J. Med. Entomol. 46, 1446–1449 (2009).
Google Scholar
-
Furlong, M. J. & Groden, E. Starvation induced stress and the susceptibility of the Colorado potato beetle, Leptinotarsa decemlineata, to infection by Beauveria bassiana. J. Invertebr. Pathol. 83, 127–138 (2003).
Google Scholar
-
Kubi, C. et al. The effect of starvation on the susceptibility of teneral and non-teneral tsetse flies to trypanosome infection. Med. Vet. Entomol. 20, 388–392 (2006).
Google Scholar
-
Le Rohellec, M. & Le Bourg, É. Contrasted effects of suppressing live yeast from food on longevity, aging and resistance to several stresses in Drosophila melanogaster. Exp. Gerontol. 44, 695–707 (2009).
Google Scholar
-
Pavlushin, S. V. et al. Effect of starvation as a population stress-factor on the activation of covert Baculovirus infection in the gypsy Moth. Biol. Bull. Rev. 11, 86–91 (2021).
Google Scholar
-
Reynolds, R. A., Kwon, H. & Smith, R. C. 20-hydroxyecdysone primes innate immune responses that limit bacterial and malarial parasite survival in Anopheles gambiae. mSphere 5, e00983-19 (2020).
Google Scholar
-
Baldridge, G. D. & Feyereisen, R. Ecdysteroid titer and oocyte growth in the northern house mosquito, Culex pipiens L.. Comp. Biochem. Physiol. A Physiol. 83, 325–329 (1986).
Google Scholar
-
Martin, D., Wang, S.-F. & Raikhel, A. S. The vitellogenin gene of the mosquito Aedes aegypti is a direct target of ecdysteroid receptor. Mol. Cell. Endocrinol. 173, 75–86 (2001).
Google Scholar
-
Wang, M. et al. Ecdysone signaling mediates the trade-off between immunity and reproduction via suppression of amyloids in the mosquito Aedes aegypti. PLoS Pathog. 18, e1010837 (2022).
Google Scholar
-
Werling, K. et al. Steroid hormone function controls non-competitive Plasmodium development in Anopheles. Cell 177, 315-325.e14 (2019).
Google Scholar
-
Hernández-Martínez, S., Rivera-Perez, C., Nouzova, M. & Noriega, F. G. Coordinated changes in JH biosynthesis and JH hemolymph titers in Aedes aegypti mosquitoes. J. Insect Physiol. 72, 22–27 (2015).
Google Scholar
-
Shapiro, A. B. et al. Juvenile hormone and juvenile hormone esterase in adult females of the mosquito Aedes aegypti. J. Insect Physiol. 32, 867–877 (1986).
Google Scholar
-
Chang, M.-M. et al. Regulation of antimicrobial peptides by juvenile hormone and its receptor, Methoprene-tolerant, in the mosquito Aedes aegypti. Insect Biochem. Mol. Biol. 128, 103509 (2021).
Google Scholar
-
Flatt, T. et al. Hormonal regulation of the humoral innate immune response in Drosophila melanogaster. J. Exp. Biol. 211, 2712–2724 (2008).
Google Scholar
-
Schwenke, R. A. & Lazzaro, B. P. Juvenile hormone suppresses resistance to infection in mated female Drosophila melanogaster. Curr. Biol. 27, 596–601 (2017).
Google Scholar
-
Clark, K. D., Kim, Y. & Strand, M. R. Plasmatocyte sensitivity to plasmatocyte spreading peptide (PSP) fluctuates with the larval molting cycle. J. Insect Physiol. 51, 587–596 (2005).
Google Scholar
-
Contreras-Garduño, J., Córdoba-Aguilar, A., Lanz-Mendoza, H. & Cordero Rivera, A. Territorial behaviour and immunity are mediated by juvenile hormone: The physiological basis of honest signalling?. Funct. Ecol. 23, 157–163 (2009).
Google Scholar
-
Rolff, J. & Siva-Jothy, M. T. Copulation corrupts immunity: A mechanism for a cost of mating in insects. Proc. Natl. Acad. Sci. 99, 9916–9918 (2002).
Google Scholar
-
Bonizzoni, M. et al. RNA-seq analyses of blood-induced changes in gene expression in the mosquito vector species, Aedes aegypti. BMC Genom. 12, 82 (2011).
Google Scholar
-
Das, S. et al. Transcriptomic and functional analysis of the Anopheles gambiae salivary gland in relation to blood feeding. BMC Genom. 11, 566 (2010).
Google Scholar
-
Hixson, B. et al. A transcriptomic atlas of Aedes aegypti reveals detailed functional organization of major body parts and gut regional specializations in sugar-fed and blood-fed adult females. eLife 11, e76132 (2022).
Google Scholar
-
Price, D. P. et al. The fat body transcriptomes of the yellow fever mosquito Aedes aegypti, pre- and post- blood meal. PLoS One 6, e22573 (2011).
Google Scholar
-
del Pilar Corena, M. et al. Carbonic anhydrase in the adult mosquito midgut. J. Exp. Biol. 208, 3263–3273 (2005).
Google Scholar
-
Graça-Souza, A. V. et al. Adaptations against heme toxicity in blood-feeding arthropods. Insect Biochem. Mol. Biol. 36, 322–335 (2006).
Google Scholar
-
Gutteridge, J. M. C. & Smith, A. Antioxidant protection by haemopexin of haem-stimulated lipid peroxidation. Biochem. J. 256, 861–865 (1988).
Google Scholar
-
Jeney, V. et al. Pro-oxidant and cytotoxic effects of circulating heme. Blood 100, 879–887 (2002).
Google Scholar
-
Oliveira, J. H. M. et al. Blood meal-derived heme decreases ROS levels in the midgut of Aedes aegypti and allows proliferation of intestinal microbiota. PLoS Pathog. 7, e1001320 (2011).
Google Scholar
-
Saeaue, L., Morales, N. P., Komalamisra, N. & Morales Vargas, R. E. Antioxidative systems defense against oxidative stress induced by blood meal in Aedes aegypti. Southeast Asian J. Trop. Med. Public Health 42, 542–549 (2011).
Google Scholar
-
Ahmed, A. M. Lipid peroxidation and oxidative protein products as biomarkers of oxidative stress in the autogenous mosquito, Aedes caspius, upon infection with the mosquitocidal bacterium, Bacillus thuringiensis Kurstaki. Pak. J. Zool. Pak. 44, 525–536 (2012).
Google Scholar
-
Chen, T.-H. et al. XBP1-mediated BiP/GRP78 upregulation copes with oxidative stress in mosquito cells during dengue 2 virus infection. BioMed. Res. Int. 2017, e3519158 (2017).
Google Scholar
-
Lindquist, S. & Craig, E. A. The heat-shock proteins. Annu. Rev. Genet. 22, 631–677 (1988).
Google Scholar
-
Santoro, M. G. Heat shock factors and the control of the stress response. Biochem. Pharmacol. 59, 55–63 (2000).
Google Scholar
-
Paim, R. M. M. et al. Functional evaluation of heat shock proteins 70 (HSP70/HSC70) on Rhodnius prolixus (Hemiptera, Reduviidae) physiological responses associated with feeding and starvation. Insect Biochem. Mol. Biol. 77, 10–20 (2016).
Google Scholar
-
Bolhassani, A. & Agi, E. Heat shock proteins in infection. Clin. Chim. Acta 498, 90–100 (2019).
Google Scholar
-
Pockley, A. G. Heat shock proteins as regulators of the immune response. Lancet 362, 469–476 (2003).
Google Scholar
-
Rosche, K. L., Sidak-Loftis, L. C., Hurtado, J., Fisk, E. A. & Shaw, D. K. Arthropods under pressure: Stress responses and immunity at the pathogen-vector interface. Front. Immunol. (2021).
-
Weng, S.-C. & Shiao, S.-H. The unfolded protein response modulates the autophagy-mediated egg production in the mosquito Aedes aegypti. Insect Mol. Biol. 29, 404–416 (2020).
Google Scholar
-
Richardson, C. E., Kooistra, T. & Kim, D. H. An essential role for XBP-1 in host protection against immune activation in C. elegans. Nature 463, 1092–1095 (2010).
Google Scholar
-
Hou, J.-N. et al. PERK signal-modulated protein translation promotes the survivability of dengue 2 virus-infected mosquito cells and extends viral replication. Viruses 9, 262 (2017).
Google Scholar
-
Vaidyanathan, R., Fleisher, A. E., Minnick, S. L., Simmons, K. A. & Scott, T. W. Nutritional stress affects mosquito survival and vector competence for West Nile virus. Vector-Borne Zoonotic Dis. 8, 727–732 (2008).
Google Scholar
-
Sarfati, M. et al. Energy costs of blood digestion in a host-specific haematophagous parasite. J. Exp. Biol. 208, 2489–2496 (2005).
Google Scholar
-
Schwenke, R. A., Lazzaro, B. P. & Wolfner, M. F. Reproduction-immunity trade-offs in insects. Annu. Rev. Entomol. 61, 239–256 (2016).
Google Scholar
-
Buttgereit, F., Burmester, G.-R. & Brand, M. D. Bioenergetics of immune functions: Fundamental and therapeutic aspects. Immunol. Today 21, 194–199 (2000).
Google Scholar
-
Lazzaro, B. P. Adenosine signaling and the energetic costs of induced immunity. PLoS Biol. 13, e1002136 (2015).
Google Scholar
-
Sheldon, B. C. & Verhulst, S. Ecological immunology: Costly parasite defences and trade-offs in evolutionary ecology. Trends Ecol. Evol. 11, 317–321 (1996).
Google Scholar
-
Adamo, S. A., Bartlett, A., Le, J., Spencer, N. & Sullivan, K. Illness-induced anorexia may reduce trade-offs between digestion and immune function. Anim. Behav. 79, 3–10 (2010).
Google Scholar
-
Ahmed, A. M., Baggott, S. L., Maingon, R. & Hurd, H. The costs of mounting an immune response are reflected in the reproductive fitness of the mosquito Anopheles gambiae. Oikos 97, 371–377 (2002).
Google Scholar
-
Ahmed, A. M. & Hurd, H. Immune stimulation and malaria infection impose reproductive costs in Anopheles gambiae via follicular apoptosis. Microbes Infect. 8, 308–315 (2006).
Google Scholar
-
Andrews, K. B., Kemper, D., Powell, K. S. & Cooper, P. D. Spatial trade-offs in the digestive and reproductive systems of grape phylloxera. Aust. J. Zool. 59, 392 (2011).
Google Scholar
-
Nordling, D., Andersson, M., Zohari, S. & Lars, G. Reproductive effort reduces specific immune response and parasite resistance. Proc. R. Soc. Lond. B Biol. Sci. 265, 1291–1298 (1998).
Google Scholar
-
Schwartz, A. & Koella, J. C. The cost of immunity in the yellow fever mosquito, Aedes aegypti depends on immune activation. J. Evol. Biol. 17, 834–840 (2004).
Google Scholar
-
Tripet, F., Aboagye-Antwi, F. & Hurd, H. Ecological immunology of mosquito–malaria interactions. Trends Parasitol. 24, 219–227 (2008).
Google Scholar
-
Berenger, J.-M. & Parola, P. Infectious Diseases 4th edn, Arthropod Vectors of Medical Importance, 104–112.e1 (Elsevier, 2017).
Google Scholar
-
Lambrechts, L. & Saleh, M.-C. Manipulating mosquito tolerance for arbovirus control. Cell Host Microbe 26, 309–313 (2019).
Google Scholar
-
Chen, Q. et al. The antenna transcriptome changes in mosquito Anopheles sinensis, pre- and post- blood meal. PLoS One 12, e0181399 (2017).
Google Scholar
-
De Das, T. et al. A synergistic transcriptional regulation of olfactory genes drives blood-feeding associated complex behavioral responses in the mosquito Anopheles culicifacies. Front. Physiol. 9, 577 (2018).
Google Scholar
-
Ni, M. et al. Screening for odorant receptor genes expressed in Aedes aegypti involved in host-seeking, blood-feeding and oviposition behaviors. Parasit. Vectors 15, 71 (2022).
Google Scholar
-
Wu, Q. et al. RNA interference of odorant receptor CquiOR114/117 affects blood-feeding behavior in Culex quinquefasciatus. Acta Trop. 204, 105343 (2020).
Google Scholar
-
Lyimo, I. N., Kessy, S. T., Mbina, K. F., Daraja, A. A. & Mnyone, L. L. Ivermectin-treated cattle reduces blood digestion, egg production and survival of a free-living population of Anopheles arabiensis under semi-field condition in south-eastern Tanzania. Malar. J. 16, 239 (2017).
Google Scholar
-
R Core Team. R: A language and environment for statistical computing (2022).
-
RStudio Team. RStudio: Integrated development environment for R (2022).
-
Ayres, J. S. & Schneider, D. S. Tolerance of infections. Annu. Rev. Immunol. 30, 271–294 (2012).
Google Scholar
-
Dunn, P. K. & Smyth, G. K. Generalized Linear Models with Examples in R 333–369 (Springer New York, 2018).
Google Scholar
-
Lenth, R.V. emmeans: Estimated Marginal Means, aka Least-Squares Means. R package version
1.7.1-1. https://CRAN.R-project.org/package=emmeans (2021).
Acknowledgements
We thank Ellen Klinger and Megan Meuti (The Ohio State University) for statistical advice and helpful experimental design comments, George Dimopoulos and Yuemei Dong (Johns Hopkins University) for providing the mCherry-transformed strain of E. coli, as well as Laura C. Harrington (Cornell University) for the gift of Ae. aegypti (Thai strain).
Funding
This work was funded by The Ohio State University Infectious Diseases Institute (https://idi.osu.edu/). Additional research support was provided by state and federal funds appropriated to The Ohio State University, College of Food, Agricultural, and Environmental Sciences (https://cfaes.osu.edu/), Ohio Agricultural Research and Development Center (https://oardc.osu.edu/). This material is also based upon work supported by the National Science Foundation Graduate Research Fellowship Program under Grant No. DGE-1343012. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Author information
Authors and Affiliations
Contributions
D.M. and S.M.S. conceived the ideas, designed the methodology, and acquired funding. D.M. and N.K.E. collected the data. D.M. and S.M.S. formally analyzed the data and led the writing of the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary Information 1.
Supplementary Information 2.
Supplementary Information 3.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
Reprints and Permissions
About this article
Cite this article
Magistrado, D., El-Dougdoug, N.K. & Short, S.M. Sugar restriction and blood ingestion shape divergent immune defense trajectories in the mosquito Aedes aegypti.
Sci Rep 13, 12368 (2023). https://doi.org/10.1038/s41598-023-39067-9
-
Received: 01 May 2023
-
Accepted: 19 July 2023
-
Published: 31 July 2023
-
DOI: https://doi.org/10.1038/s41598-023-39067-9
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.