Congenital disorders
USPSTF Review: Folic Acid Supplementation to Prevent Neural Tube Defects
Importance
Neural tube defects are among the most common birth defects in the US.
Objective
To review new evidence on the benefits and harms of folic acid supplementation for the prevention of neural tube defects to inform the US Preventive Services Task Force.
Evidence Review
Sources included PubMed, Cochrane Library, Embase, and trial registries from July 1, 2015, through July 2, 2021; references; and experts, with surveillance through February 10, 2023. Two investigators independently reviewed English-language randomized studies and nonrandomized cohort studies in very highly developed countries that focused on the use of folic acid supplementation for the prevention of neural tube defect–affected pregnancies; methodological quality was dually and independently assessed.
Findings
Twelve observational studies (reported in 13 publications) were eligible for this limited update (N = 1 244 072). Of these, 3 studies (n = 990 372) reported on the effect of folic acid supplementation on neural tube defects. For harms, 9 studies were eligible: 1 randomized clinical trial (n = 431) reported on variations in twin delivery, 7 observational studies (n = 761 125) reported on the incidence of autism spectrum disorder, and 1 observational study (n = 429 004) reported on maternal cancer. Two cohort studies and 1 case-control study newly identified in this update reported on the association between folic acid supplementation and neural tube defects (n = 990 372). One cohort study reported a statistically significant reduced risk of neural tube defects associated with folic acid supplementation taken before pregnancy (adjusted relative risk [aRR], 0.54 [95% CI, 0.31-0.91]), during pregnancy (aRR, 0.62 [95% CI, 0.39-0.97]), and before and during pregnancy (aRR, 0.49 [95% CI, 0.29-0.83]), but this association occurred for only the later of 2 periods studied (2006-2013 and not 1999-2005). No other statistically significant benefits were reported overall. No study reported statistically significant harms (multiple gestation, autism, and maternal cancer) associated with pregnancy-related folic acid exposure.
Conclusions and Relevance
New evidence from observational studies provided additional evidence of the benefit of folic acid supplementation for preventing neural tube defects and no evidence of harms related to multiple gestation, autism, or maternal cancer. The new evidence was consistent with previously reviewed evidence on benefits and harms.
Introduction
Neural tube defects are major congenital malformations often caused by low folate concentrations in the body at the time of conception. These defects frequently result in significant disability or death for affected fetuses and children. Strategies that enhance folic acid uptake before pregnancy offer the best chance of prevention.
In 2017, the US Preventive Services Task Force (USPSTF) concluded that folic acid supplementation in the periconceptional period has substantial benefits in reducing the risk of neural tube defects in the developing fetus1 and reaffirmed its 2009 recommendation that all persons who are planning or capable of pregnancy take a daily supplement containing 0.4 to 0.8 mg (400-800 μg) of folic acid (A recommendation). The 2017 USPSTF recommendation was based on previously reviewed evidence from a randomized clinical trial and observational studies reporting reduced neural tube defects with supplementation and no consistent evidence of harms such as multiple gestation, maternal adverse effects, or child respiratory illness.
This limited evidence update aimed to identify studies published since the previous (2017) evidence review2 conducted for the USPSTF to inform a reaffirmation of the current recommendation.
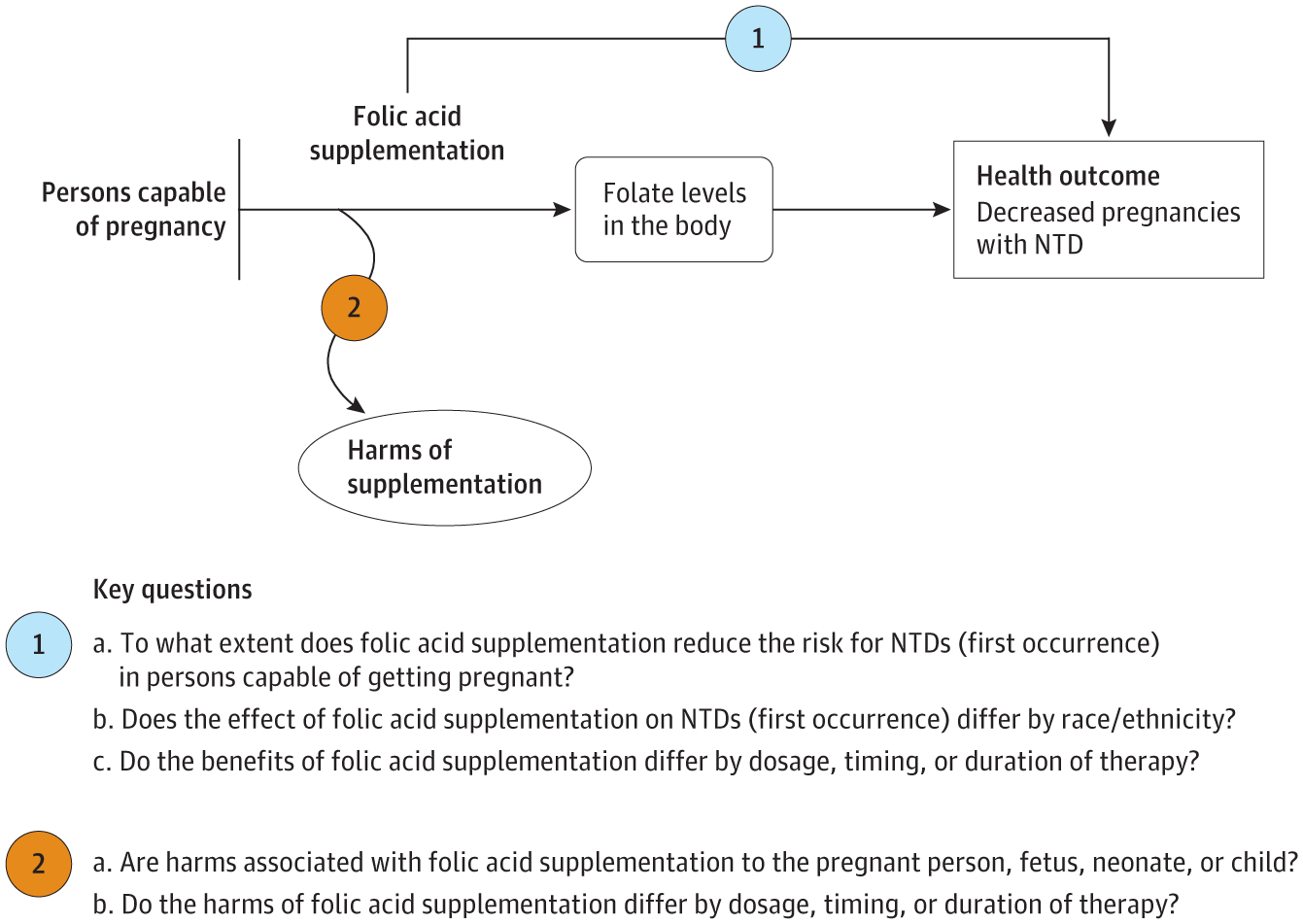
An analytic framework and 2 key questions guided the limited evidence update (Figure). A literature search of PubMed/MEDLINE, the Cochrane Library, Embase, and trial registries was conducted from July 1, 2015, through July 2, 2021. Additional sources included reference lists of retrieved articles, outside experts, and public commenters, with ongoing surveillance of the literature through February 10, 2023. Two investigators independently evaluated the eligibility of all abstracts and articles and rated study quality using predefined criteria.4 Detailed methods and results are available in the full evidence report.4
English-language randomized and nonrandomized studies that focused on the use of folic acid supplementation (by itself or in multivitamins) for the prevention of neural tube defect–affected pregnancies in persons capable of getting pregnant were eligible. Studies conducted in very highly developed countries and that investigated potential harms of folic acid supplementation, such as maternal cancer and autism spectrum disorder, were also eligible. Ineligible studies included poor-quality studies and those focusing solely on persons taking antiseizure medications or with a history of neural tube defects in previous pregnancies.
Twelve observational studies (reported in 13 publications5-17) (Table) were eligible for this limited update (N = 1 244 072 [from nonoverlapping cohorts]). Of these, 3 studies (n = 990 372) assessed the effect of folic acid supplementation on neural tube defects.5-8 No studies examined differences by race or ethnicity. For harms, 9 studies were eligible; 1 randomized clinical trial (n = 431) assessed variations in twin delivery,9 7 observational studies (n = 761 125) examined the incidence of autism spectrum disorders,10-16 and 1 observational study (n = 429 004) reported on maternal cancer.17 The Table also reports details on studies from the 2017 evidence review.18-47
Benefits of Folic Acid Supplementation
Regarding the benefits of folic acid supplementation, 2 cohort studies and 1 case-control study in this update examined the association between folic acid supplementation and neural tube defects (n = 990 372).5-8 Food fortification and supplementation practices varied by setting. Of these studies, 1 cohort study set in Norway (no mandatory fortification) reported on neural tube defects among live births and stillborn infants from 1999 to 2013 overall and also stratified results into 2 separate periods: 1999 to 2005 and 2006 to 2013.6 The authors performed this stratified analysis because they found that the overall adjusted relative risk (aRR) was affected by year of birth. Several external events of importance were cited to explain differences by period: the introduction of folic acid recommendations in 1999, inclusion of 0.2 mg of folic acid in multivitamin supplements from 2004 onward (before 2004, most multivitamins did not include folic acid), and increased adherence to folic acid recommendations in the second half of the period analyzed (2006-2013).6 The authors reported no statistically significant benefits in the first of the 2 periods (1999-2005), regardless of timing of supplementation (before pregnancy, during pregnancy, or before and during pregnancy). In contrast, in the second period (2006-2013), the authors reported a statistically significant reduced risk of neural tube defects associated with folic acid supplementation taken before pregnancy (aRR, 0.54 [95% CI, 0.31-0.91]), during pregnancy (aRR, 0.62 [95% CI, 0.39-0.97]), and before and during pregnancy (aRR, 0.49 [95% CI, 0.29-0.83]).6
The second cohort study, set in Japan (no mandatory food fortification), reported no statistically significant differences associated with adequate (preconception) folic acid supplementation (adjusted odds ratio [aOR], 0.62 [95% CI, 0.23-1.71]) when compared with inadequate use (use after pregnancy recognition or no use).5 The third study, a case-control study set in the US and Canada in the period following food fortification, reported on participants with pregestational diabetes and prepregnancy obesity.8 The study reported that cases occurred more often among persons with unplanned pregnancies.8 Authors reported a statistically significant reduction in neural tube defects in women with prepregnancy obesity taking 0.4 mg to 1 mg of folic acid, when compared with women taking no supplementation and adjusting for maternal age (aOR, 0.54 [95% CI, 0.29-0.95]).8 Results adjusting for planned pregnancy rather than maternal age were similar but not statistically significant (aOR, 0.57 [95% CI, 0.30-1.02]).8 Across all 3 studies, no other statistically significant benefits were reported overall or by dose (1 study8) or timing (1 study6,7).
Harms of Folic Acid Supplementation
No study of harms (multiple gestation, autism, and maternal cancer) reported significant associations with pregnancy-related folic acid exposure.9-17
Discussion
This evidence review identified 3 new observational studies reporting on the association between folic acid supplementation before or during pregnancy and neural tube defects in offspring. Mandatory food fortification and supplementation practices varied by geography and period of investigation and contributed to heterogeneity across studies. Nevertheless, these new studies provided additional evidence of the benefit of folic acid supplementation for preventing neural tube defects. Nine new observational studies found no evidence of harms related to multiple gestation, autism, or maternal cancer. This new evidence is consistent with previously reviewed evidence on the benefits and harms of folic acid supplementation to prevent neural tube defects.
Accepted for Publication: June 19, 2023.
Concept and design: Viswanathan, Urrutia, Kahwati.
Acquisition, analysis, or interpretation of data: Viswanathan, Urrutia, Hudson, Middleton.
Drafting of the manuscript: Viswanathan, Urrutia, Hudson, Middleton.
Statistical analysis: Viswanathan.
Obtained funding: Viswanathan, Kahwati.
Administrative, technical, or material support: Viswanathan, Hudson, Middleton, Kahwati.
Supervision: Viswanathan.
Conflict of Interest Disclosures: None reported.
Funding/Support: This research was funded under contract 75Q80120D00007, Task Order 01, from the Agency for Healthcare Research and Quality (AHRQ), US Department of Health and Human Services, under a contract to support the USPSTF.
Role of the Funder/Sponsor: Investigators worked with USPSTF members and AHRQ staff to develop the scope, analytic framework, and key questions for this review. AHRQ had no role in study selection, quality assessment, or synthesis. AHRQ staff provided project oversight, reviewed the report to ensure the analysis met methodological standards, and distributed the draft for peer review. Otherwise, AHRQ had no role in the conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript findings. The opinions expressed in this document are those of the authors and do not represent the official position of AHRQ or the US Department of Health and Human Services.
Additional Contributions: We thank the following individuals for their contributions to this project: Justin Mills, MD, MPH (AHRQ medical officer); Tina Fan, MD, MPH (previous associate scientific director, AHRQ); and Tracy Wolff, MD, MPH (scientific director, AHRQ USPSTF program); current and former members of the USPSTF; peer and federal partner reviewers; RTI International–University of North Carolina Evidence-based Practice Center staff: Christiane Voisin, MSLS (research librarian); Roberta Wines, MPH, and Carol Woodell, BSPH (current and former Evidence-based Practice Center program managers); Nila Sathe, MA, MLIS (quality assurance); Sharon Barrell, MA (editor); and Teyonna Downing and Alex Cone (publications specialists). USPSTF members, peer reviewers, and federal partner reviewers did not receive financial compensation for their contributions.
Additional Information: A draft version of the full evidence report underwent external peer review from 3 content experts (Nancy Rose, MD, University of Utah; Jorge Chavarro, MD, ScD, Harvard University; Kimberly Gregory, MD, MPH, Cedars-Sinai Medical Center) and 3 individuals from 2 federal partners (Centers for Disease Control and Prevention, National Institutes of Health). Comments from reviewers were presented to the USPSTF during its deliberation of the evidence and were considered in preparing the final evidence review.
Editorial Disclaimer: This evidence report is presented as a document in support of the accompanying USPSTF recommendation statement. It did not undergo additional peer review after submission to JAMA.
References
Viswanathan
M, Treiman
KA, Kish-Doto
J, Middleton
JC, Coker-Schwimmer
EJL, Nicholson
WK. Folic Acid Supplementation: An Evidence Review for the US Preventive Services Task Force. Evidence Synthesis No. 145. Agency for Healthcare Research and Quality; 2017. AHRQ publication 14-05214-EF-1.
Viswanathan
M, Urrutia
RP, Hudson
KN, Middleton
JC, Kahwati
LC. Folic Acid Supplementation to Prevent Neural Tube Defects: A Limited Systematic Review Update for the US Preventive Services Task Force. Evidence Synthesis No. 230. Agency for Healthcare Research and Quality; 2023. AHRQ publication 22-05302-EF-1.
H, Obara
T, Nishigori
T,
et al; Japan Environment & Children’s Study Group. Preconception folic acid supplementation use and the occurrence of neural tube defects in Japan: a nationwide birth cohort study of the Japan Environment and Children’s Study. Congenit Anom (Kyoto). 2019;59(4):110-117. doi:10.1111/cga.12293PubMedGoogle ScholarCrossref
T, Øyen
N, Klungsøyr
K, Nilsen
RM, Daltveit
AK, Vollset
SE. Maternal use of folic acid supplements and infant risk of neural tube defects in Norway 1999-2013. Scand J Public Health. 2016;44(6):619-626. doi:10.1177/1403494816649494PubMedGoogle ScholarCrossref
T, Bjørge
T, Haaland
ØA, Klungsøyr
K, Vollset
SE, Øyen
N. Maternal use of folic acid and multivitamin supplements and infant risk of birth defects in Norway, 1999-2013. Br J Nutr. 2020;124(3):316-329. doi:10.1017/S0007114520001178PubMedGoogle ScholarCrossref
JM, Parker
SE, Benedum
CM, Mitchell
AA, Tinker
SC, Werler
MM. Periconceptional folic acid and risk for neural tube defects among higher risk pregnancies. Birth Defects Res. 2019;111(19):1501-1512. doi:10.1002/bdr2.1579PubMedGoogle ScholarCrossref
R, Filippini
F, Cipriani
S,
et al. Efficacy of 4.0 mg versus 0.4 mg folic acid supplementation on the reproductive outcomes: a randomized controlled trial. Nutrients. 2021;13(12):4422. doi:10.3390/nu13124422PubMedGoogle ScholarCrossref
M, Granström
C, Lyall
K, Ascherio
A, Olsen
SF. Research letter: folic acid supplementation and intake of folate in pregnancy in relation to offspring risk of autism spectrum disorder. Psychol Med. 2018;48(6):1048-1054. doi:10.1017/S0033291717002410PubMedGoogle ScholarCrossref
J, Liew
Z, Olsen
J, Nohr
EA, Catov
JM, Ritz
B. Preconceptional and prenatal supplementary folic acid and multivitamin intake and autism spectrum disorders. Autism. 2016;20(6):710-718. doi:10.1177/1362361315604076PubMedGoogle ScholarCrossref
EA, Magnusson
C, Gardner
RM,
et al. Antenatal nutritional supplementation and autism spectrum disorders in the Stockholm youth cohort: population based cohort study. BMJ. 2017;359:j4273. doi:10.1136/bmj.j4273PubMedGoogle ScholarCrossref
RM, Surén
P, Gunnes
N,
et al. Analysis of self-selection bias in a population-based cohort study of autism spectrum disorders. Paediatr Perinat Epidemiol. 2013;27(6):553-563. doi:10.1111/ppe.12077PubMedGoogle ScholarCrossref
SZ, Kodesh
A, Viktorin
A,
et al. Association of maternal use of folic acid and multivitamin supplements in the periods before and during pregnancy with the risk of autism spectrum disorder in offspring. JAMA Psychiatry. 2018;75(2):176-184. doi:10.1001/jamapsychiatry.2017.4050PubMedGoogle ScholarCrossref
S, Davidovitch
M, Rotem
RS, Chodick
G, Shalev
V, Koren
G. High dose folic acid during pregnancy and the risk of autism: the birth order bias: a nested case-control study. Reprod Toxicol. 2019;89:173-177. doi:10.1016/j.reprotox.2019.07.083PubMedGoogle ScholarCrossref
AE, Dobó
M, Vargha
P. Hungarian cohort-controlled trial of periconceptional multivitamin supplementation shows a reduction in certain congenital abnormalities. Birth Defects Res A Clin Mol Teratol. 2004;70(11):853-861. doi:10.1002/bdra.20086PubMedGoogle ScholarCrossref
MM, Shapiro
S, Mitchell
AA. Periconceptional folic acid exposure and risk of occurrent neural tube defects. JAMA. 1993;269(10):1257-1261. PubMedGoogle ScholarCrossref
JL, Rhoads
GG, Simpson
JL,
et al; National Institute of Child Health and Human Development Neural Tube Defects Study Group. The absence of a relation between the periconceptional use of vitamins and neural-tube defects. N Engl J Med. 1989;321(7):430-435. doi:10.1056/nejm198908173210704PubMedGoogle ScholarCrossref
L, Hendricks
KA, Cooper
SP, Sweeney
AM, Hardy
RJ, Larsen
RD. Neural tube defects among Mexican Americans living on the US-Mexico border: effects of folic acid and dietary folate. Am J Epidemiol. 2000;152(11):1017-1023. doi:10.1093/aje/152.11.1017PubMedGoogle ScholarCrossref
AJ, Tinker
SC, Lupo
PJ, Canfield
MA, Mitchell
LE; National Birth Defects Prevention Study. Proportion of neural tube defects attributable to known risk factors. Birth Defects Res A Clin Mol Teratol. 2013;97(1):42-46. doi:10.1002/bdra.23100PubMedGoogle ScholarCrossref
BS, Cleves
MA, Siega-Riz
AM,
et al. Neural tube defects and maternal folate intake among pregnancies conceived after folic acid fortification in the United States. Am J Epidemiol. 2009;169(1):9-17. doi:10.1093/aje/kwn331PubMedGoogle ScholarCrossref
AE, Métneki
J, Dudás
I. The higher rate of multiple births after periconceptional multivitamin supplementation: an analysis of causes. Acta Genet Med Gemellol (Roma). 1994;43(3-4):175-184. doi:10.1017/S0001566000001938PubMedGoogle ScholarCrossref
R, Heron
J, Lewis
S, Davey Smith
G, Sterne
JA, Henderson
J. The association between mother and child MTHFR C677T polymorphisms, dietary folate intake and childhood atopy in a population-based, longitudinal birth cohort. Clin Exp Allergy. 2008;38(2):320-328. doi:10.1111/j.1365-2222.2007.02902.xPubMedGoogle ScholarCrossref
JC, Timmermans
S, Jaddoe
VW,
et al. High circulating folate and vitamin B-12 concentrations in women during pregnancy are associated with increased prevalence of atopic dermatitis in their offspring. J Nutr. 2012;142(4):731-738. doi:10.3945/jn.111.154948PubMedGoogle ScholarCrossref
FJ, Mommers
M, Penders
J, Smits
L, Thijs
C. Folic acid use in pregnancy and the development of atopy, asthma, and lung function in childhood. Pediatrics. 2011;128(1):e135-144. doi:10.1542/peds.2010-1690PubMedGoogle ScholarCrossref
MJ, Moore
VM, Rumbold
AR, Davies
MJ. Effect of supplemental folic acid in pregnancy on childhood asthma: a prospective birth cohort study. Am J Epidemiol. 2009;170(12):1486-1493. doi:10.1093/aje/kwp315PubMedGoogle ScholarCrossref
KS, Cordero
AM, Qi
YP, Mulinare
J, Dowling
NF, Berry
RJ. Prenatal folic acid and risk of asthma in children: a systematic review and meta-analysis. Am J Clin Nutr. 2013;98(5):1272-1281. doi:10.3945/ajcn.113.065623PubMedGoogle Scholar
AE, Dudás
I, Métneki
J. Pregnancy outcomes in a randomised controlled trial of periconceptional multivitamin supplementation: final report. Arch Gynecol Obstet. 1994;255(3):131-139. doi:10.1007/BF02390940PubMedGoogle Scholar