Congenital disorders
Association between antenatal corticosteroids and risk of serious infection in children: nationwide cohort study
Abstract
Objective To investigate the associations between exposure to antenatal corticosteroids and serious infection in children during the first three, six, and 12 months of life.
Design Nationwide cohort study.
Setting National Health Insurance Research Database, Birth Reporting Database, and Maternal and Child Health Database, 1 January 2008 to 31 December 2019, to identify all pregnant individuals and their offspring in Taiwan.
Participants 1 960 545 pairs of pregnant individuals and their singleton offspring. 45 232 children were exposed and 1 915 313 were not exposed to antenatal corticosteroids.
Main outcome measures Incidence rates were estimated for overall serious infection, sepsis, pneumonia, acute gastroenteritis, pyelonephritis, meningitis or encephalitis, cellulitis or soft tissue infection, septic arthritis or osteomyelitis, and endocarditis during the first three, six, and 12 months of life in children exposed versus those not exposed to antenatal corticosteroids. Cox proportional hazards models were performed to quantify adjusted hazard ratios with 95% confidence intervals for each study outcome.
Results The study cohort was 1 960 545 singleton children: 45 232 children were exposed to one course of antenatal corticosteroids and 1 915 313 children were not exposed to antenatal corticosteroids. The adjusted hazard ratios for overall serious infection, sepsis, pneumonia, and acute gastroenteritis among children exposed to antenatal corticosteroids were significantly higher than those not exposed to antenatal corticosteroids during the first six months of life (adjusted hazard ratio 1.32, 95% confidence interval 1.18 to 1.47, P<0.001, for overall serious infection; 1.74, 1.16 to 2.61, P=0.01, for sepsis; 1.39, 1.17 to 1.65, P<0.001, for pneumonia; and 1.35, 1.10 to 1.65, P<0.001, for acute gastroenteritis).Similarly, the adjusted hazard ratios for overall serious infection (P<0.001), sepsis (P=0.02), pneumonia (P<0.001), and acute gastroenteritis (P<0.001) were significantly higher from birth to 12 months of life. In the sibling matched cohort, the results were comparable with those observed in the whole cohort, with a significantly increased risk of sepsis in the first six (P=0.01) and 12 (P=0.04) months of life.
Conclusions This nationwide cohort study found that children exposed to one course of antenatal corticosteroids were significantly more likely to have an increased risk of serious infection during the first 12 months of life. These findings suggest that before starting treatment, the long term risks of rare but serious infection associated with antenatal corticosteroids should be carefully weighed against the benefits in the perinatal period.
Introduction
One course of antenatal corticosteroids is considered the standard of care for pregnant individuals between 24 and 34 weeks of gestation at risk of preterm delivery to prevent neonatal respiratory distress syndrome.12345 With the increasing use of antenatal corticosteroids in pregnancies at risk of preterm delivery, real world studies on the long term safety of this treatment beyond the neonatal period are needed.6
Synthetic corticosteroids, such as betamethasone and dexamethasone, readily cross the placenta when given during the antenatal period and expose the fetus to supraphysiological levels of corticosteroids.78 Although the lungs are the primary target of antenatal corticosteroids, other organ systems, such as the neurological and immune systems, might also be affected. Recent observational cohort studies suggest that exposure to antenatal corticosteroids could be associated with long term neurodevelopmental harms in children.910 Another concern is the potential risk of rare but serious infection in children exposed to antenatal corticosteroids.111213 Recent nationwide studies in the US and Taiwan have reported increased risks of serious infections, such as sepsis and pneumonia, after short courses of oral corticosteroids in the general population.141516 These studies, however, did not evaluate the transgenerational effect of exposure to antenatal corticosteroids in infants, who might be at higher risk of serious infection.
A better understanding of the risk of serious infection in children exposed to antenatal corticosteroids to accelerate fetal lung maturation before birth is needed. Lack of sufficiently large sample sizes, however, is a critical barrier for randomised controlled trials to examine the risks of rare adverse events, such as serious infection.17 Although evidence for an increased risk of serious infection, such as neonatal sepsis and congenital pneumonia, after exposure to antenatal corticosteroids has emerged in recent years,111213 rigorous data focusing on the potential harms of antenatal corticosteroids in children in large population based cohorts is lacking. In this nationwide cohort study based on the whole of the National Health Insurance Research Database in Taiwan, we aimed to quantify the association between antenatal corticosteroids and the risk of rare but important serious infection in children during the first few months of life.
Methods
Data sources and study cohort
In this nationwide cohort study, we used data from the National Health Insurance Research Database, Birth Reporting Database, and Maternal and Child Health Database to identify all pregnant individuals and their offspring in Taiwan between 2008 and 2019. About 23 million patients representing nearly 99.9% of the Taiwanese population are covered by the National Health Insurance Programme. A detailed description of the National Health Insurance Research Database has been previously published, and diagnostic records, medication use, and use of healthcare facilities have been validated.1819 In this study, we used de-identified medical claims records and prescription data from the National Health Insurance Research Database, Birth Reporting Database, and Maternal and Child Health Database.
We identified pairs of pregnant individuals and their offspring between 1 January 2008 and 31 December 2019. Exclusion criteria were pregnancies resulting in multiple births, pregnancies with more than one course of antenatal corticosteroids, and pregnancies resulting in offspring with congenital anomalies or catastrophic illnesses. eFigure 1 outlines the identification criteria for the study cohort. To construct a sibling matched cohort, we excluded families with only one child born in 2008-19.
Exposure to antenatal corticosteroids
Information on exposure to antenatal corticosteroids (yes/no) was obtained from the prescription records of the National Health Insurance Research Database. The most commonly used antenatal corticosteroids in Taiwan are betamethasone (two 12 mg doses intramuscularly 24 hours apart) and dexamethasone (four 6 mg doses intramuscularly 12 hours apart), according to the recommendations of the American College of Obstetricians and Gynecologists.2 Only one course of antenatal corticosteroids prescribed by obstetricians was included in the analyses.
Study outcomes
The outcomes of interest in this study were hospital admission of children during the first three, six, and 12 months of life because of serious infection, specifically sepsis, pneumonia, acute gastroenteritis, pyelonephritis, meningitis or encephalitis, cellulitis or soft tissue infection, septic arthritis or osteomyelitis, and endocarditis. We used ICD-9-CM (international classification of diseases, ninth revision, clinical modification) codes before 2015 and ICD-10-CM (10th revision) codes after 2015 to identify each type of serious infection from the primary inpatient diagnoses (eTable 1).1617 Overall serious infection was defined as admission to hospital for any of the serious infections (sepsis, pneumonia, acute gastroenteritis, pyelonephritis, meningitis or encephalitis, cellulitis or soft tissue infection, septic arthritis or osteomyelitis, or endocarditis). In this study, we included serious infections that occurred after hospital discharge after birth. Episodes of intussusception were treated as a negative control endpoint (eTable 1).
Propensity score analysis
We applied a propensity score method, inverse probability of treatment weighting (IPTW), to account for the systematic differences at baseline between children exposed and not exposed to antenatal corticosteroids in the whole cohort and in the sibling matched cohort.2021 The propensity score was estimated based on the predicted probability of each child exposed to antenatal corticosteroids conditional on their baseline personal and clinical conditions. For each child, exposure to antenatal corticosteroids during pregnancy (yes/no) was regressed on potential confounding factors in a logistic regression model. Potential confounding factors were maternal age at delivery, parity, mode of delivery, premature rupture of membranes, gestational diabetes, gestational hypertension, maternal infectious disorders before and during pregnancy, maternal mental disorders at any time, maternal asthma, maternal chronic obstructive pulmonary disease, maternal immunological disorders (eg, systemic lupus erythematosus and rheumatoid arthritis), substance abuse, socioeconomic status, child’s gestational age at birth, sex, birth weight, and Apgar score (maximum at one and five minutes), birth year, number of siblings, number of inpatient visits, and number of outpatient visits. IPTW with propensity score was adjusted in the subsequent analyses. We used ICD-9-CM codes before 2015 and ICD-10-CM codes after 2015 to identify the physical and mental conditions included in the propensity score calculations (eTable 2).
Statistical analysis
We used the standardised mean difference to evaluate differences in characteristics at baseline between children exposed and not exposed to antenatal corticosteroids. A standardised mean difference of <0.1 was considered as a negligible difference between two groups.22 We evaluated the effect of antenatal corticosteroids on serious infection in three follow-up periods: the first three, six, and 12 months of life, with date of birth as the index date. We computed incidence rates per 1000 person years for each type of serious infection for children exposed and not exposed to antenatal corticosteroids. For the whole study cohort of children, we used Cox proportional hazards models with IPTW to account for the systematic baseline differences and the robust variance estimator to correct for intraindividual dependence to quantify crude hazard ratios with 95% confidence intervals for each study outcome.
Adjusted models were also constructed and adjusted hazard ratios with 95% confidence interval were reported. The list of covariates were maternal age at delivery, parity, mode of delivery, premature rupture of membranes, gestational diabetes, gestational hypertension, maternal infectious disorders before and during pregnancy, maternal mental disorders at any time, maternal asthma, maternal chronic obstructive pulmonary disease, maternal immunological disorders (eg, systemic lupus erythematosus and rheumatoid arthritis), substance abuse, socioeconomic status, child’s gestational age at birth, sex, birth weight, and Apgar score (maximum at one and five minutes), birth year, number of siblings, number of inpatient visits, and number of outpatient visits.
Kaplan-Meier plots were generated to estimate the cumulative incidence of serious infection between children exposed and not exposed to antenatal corticosteroids in the first 12 months of life. We used the log rank test to test differences between the two groups. We performed sensitivity analyses with alternative definitions of serious infection: two outpatient visits or one hospital admission with a primary diagnosis of serious infection; and excluding infants born to pregnant individuals with premature rupture of membranes, gestational diabetes, or gestational hypertension. Subgroup analyses using Cox proportional hazards models with consideration of IPTW and the robust variance estimator were performed to examine the potential confounding or modifying effects of: children born at full term versus those born preterm (ie, full term infants exposed to antenatal corticosteroids compared with full term infants not exposed; and preterm infants exposed to antenatal corticosteroids compared with preterm infants not exposed); use of antenatal corticosteroids at gestational age <34 weeks versus ≥34 weeks; and two types of antenatal corticosteroids, betamethasone and dexamethasone. To further explore the effect of timing of exposure to antenatal corticosteroids, we carried out a fine grained analysis based on the categories of Alenius et al (gestational age ≤27 weeks, 28-31 weeks, 32-33 weeks, and ≥34 weeks).23
In the sibling matched cohort, we applied stratified Cox proportional hazards models with IPTW and a separate group for each family to control for unmeasured shared genetic and environmental factors among siblings. Cox proportional hazards assumptions were examined by plotting Kaplan-Meier curves with survival function versus follow-up time, and by including product terms between the predictors and function of survival time in the models. Parallel Kaplan-Meier curves were found (eFigures 2-4); no significant product terms were observed in the models, indicating that the assumptions were held. A P value <0.05 was considered to be significant. All analyses were conducted with SAS version 9.4 (SAS Institute, Cary, NC).
Patient and public involvement
Patients and the public were not involved in the research question, study design, interpreting the results, or writing the manuscript, owing to the retrospective and de-identified nature of the data as well as National Health Insurance Research Database privacy restrictions.
Results
Baseline characteristics of the study participants
The study cohort was 1 960 545 singleton children born between 2008 and 2019; 45 232 children were exposed and 1 915 313 were not exposed to antenatal corticosteroids. Of the 45 232 children exposed to antenatal corticosteroids, 18 148 (40.1%) were born at full term and 27 084 (59.9%) were born preterm. Table 1 shows the baseline characteristics of the pregnant individuals and their offspring in the study cohort. After applying IPTW, all baseline characteristics were similar between the two groups (table 1). The rates of antibiotic use not associated with hospital admission were 13.7% and 12.1% in children exposed versus not exposed to antenatal corticosteroids, respectively. The prevalence of neonatal Cushing syndrome was 6.63×10−5 and 6.16×10−5 in the two groups, respectively.
Baseline characteristics of the study cohort
Association between antenatal corticosteroids and serious infection
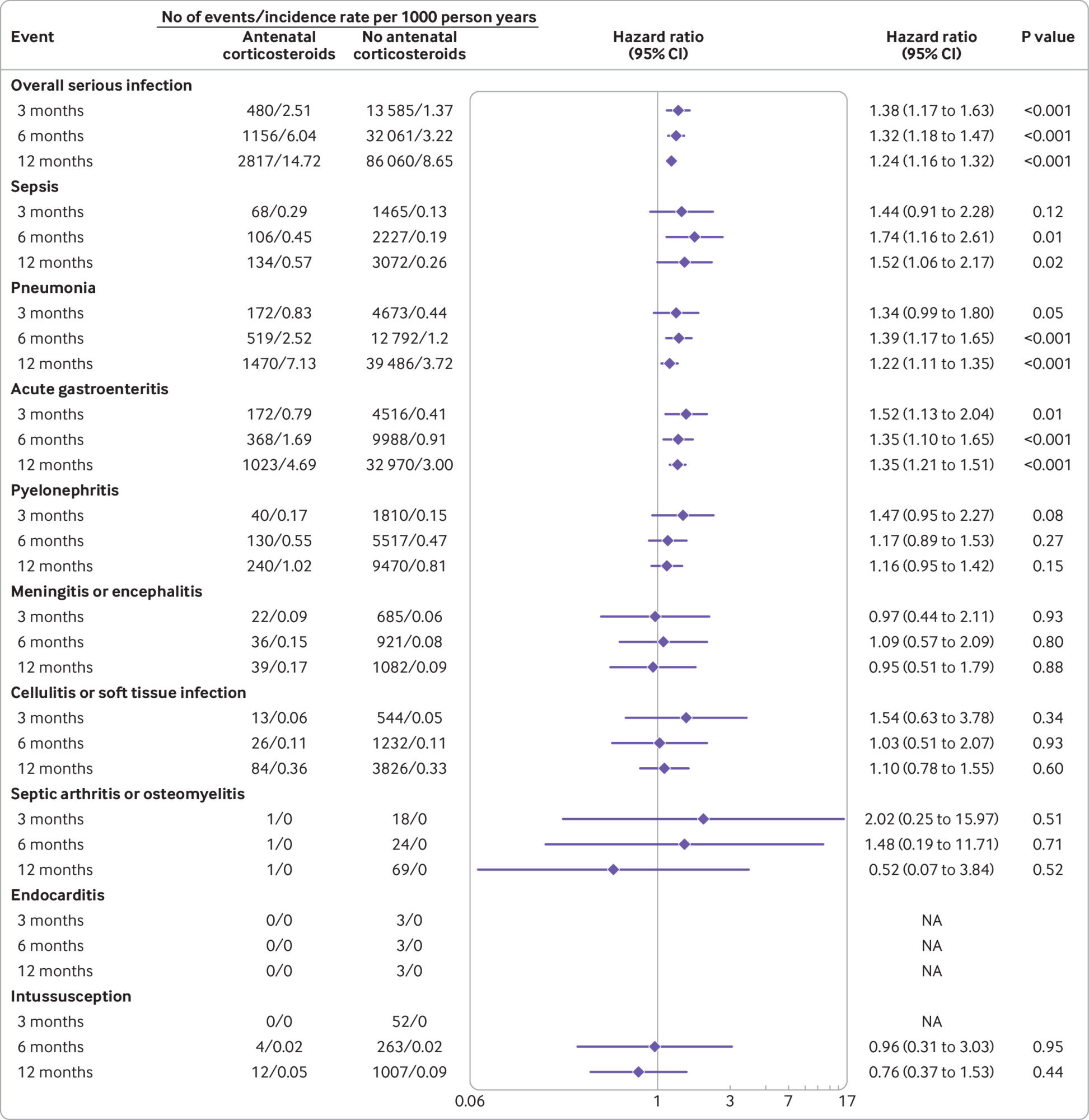
We evaluated the effect of antenatal corticosteroids on serious infection in three follow-up periods: the first three, six, and 12 months of life. Figure 1 shows that the adjusted hazard ratios for serious infection during the first six months of life among children exposed to antenatal corticosteroids were significantly higher than those not exposed to antenatal corticosteroids: adjusted hazard ratio 1.32, 95% confidence interval 1.18 to 1.47, P<0.001, for overall serious infection; 1.74, 1.16 to 2.61, P=0.01, for sepsis; 1.39, 1.17 to 1.65, P<0.001, for pneumonia; and 1.35, 1.10 to 1.65, P<0.001, for acute gastroenteritis. The adjusted hazard ratios for overall serious infection (P<0.001), sepsis (P=0.02), pneumonia (P<0.001), and acute gastroenteritis (P<0.001) were also significantly higher from birth to 12 months of life (fig 1). We found no association between exposure to antenatal corticosteroids and the risk of intussusception, the negative control endpoint (fig 1). The cumulative incidence of overall serious infection (P<0.001), sepsis (P<0.001), pneumonia (P<0.001), acute gastroenteritis (P<0.001), and pyelonephritis (P=0.03), separately, during the first 12 months of life, was greater in children exposed to antenatal corticosteroids than in those not exposed (fig 2).
Association between antenatal corticosteroids and serious infection in children. Hazard ratio was obtained from adjusted models controlled for the covariates: maternal age at delivery, parity, mode of delivery, premature rupture of membranes, gestational diabetes, gestational hypertension, maternal infectious disorders before and during pregnancy, maternal mental disorders at any time, maternal asthma, maternal chronic obstructive pulmonary disease, maternal immunological disorders (eg, systemic lupus erythematosus and rheumatoid arthritis), substance abuse, socioeconomic status, child’s gestational age at birth, sex, birth weight, and Apgar score (maximum at one and five minutes), birth year, number of siblings, number of inpatient visits, and number of outpatient visits. CI=confidence interval; NA=not available
“>
Association between antenatal corticosteroids and serious infection in children. Hazard ratio was obtained from adjusted models controlled for the covariates: maternal age at delivery, parity, mode of delivery, premature rupture of membranes, gestational diabetes, gestational hypertension, maternal infectious disorders before and during pregnancy, maternal mental disorders at any time, maternal asthma, maternal chronic obstructive pulmonary disease, maternal immunological disorders (eg, systemic lupus erythematosus and rheumatoid arthritis), substance abuse, socioeconomic status, child’s gestational age at birth, sex, birth weight, and Apgar score (maximum at one and five minutes), birth year, number of siblings, number of inpatient visits, and number of outpatient visits. CI=confidence interval; NA=not available
“>
Antenatal corticosteroids and probability of overall serious infection, sepsis, pneumonia, acute gastroenteritis, and pyelonephritis in children during the first 12 months of life
Sensitivity analyses
Sensitivity analyses were performed to investigate the effect of different definitions of study outcomes: infants with alternative definitions of serious infection (ie, two outpatient visits or one hospital admission with a primary diagnosis of serious infection); and excluding infants born to pregnant individuals with premature rupture of membranes, gestational diabetes, or gestational hypertension. We found that the results of the sensitivity analyses (eTables 3-4) were comparable with the main results.
Subgroup and fine grained analyses
In subgroup analyses grouped by gestational age (full term versus preterm), similar results were found in children born at full term compared with the whole cohort (table 2). In the preterm group, we found significantly higher hazard ratios for overall serious infection (P<0.001), pneumonia (P=0.001), and acute gastroenteritis (P=0.001) during the first 12 months of life (table 2).
Association between use of antenatal corticosteroids and serious infection in children born at full term versus preterm
We also conducted subgroup analyses grouped by gestational age when exposed to antenatal corticosteroids and by different corticosteroids. Similar risks were found in children exposed to antenatal corticosteroids at gestational age <34 weeks versus ≥34 weeks (eTable 5). Results from the fine grained analysis were comparable with the main results (eTable 6). We also found similar results in those exposed to betamethasone versus dexamethasone (eTable 7).
Sibling matched analysis
In the sibling matched cohort, 506 071 families were included, with 24 791 discordant and 605 146 concordant sibling pairs. Figure 3 shows a significant increase in the risk of sepsis in the first six and 12 months of life (adjusted hazard ratio 2.00, 95% confidence interval 1.17 to 3.42, P=0.01, and 1.65, 1.02 to 2.69, P=0.04, respectively), similar to the results in the whole cohort.
“>
Association between antenatal corticosteroids and serious infection in sibling matched analysis. Hazard ratio was obtained from adjusted models controlled for the covariates: child’s gestational age at birth, sex, birth weight, and Apgar score (maximum at one and five minutes), and birth order number. CI=confidence interval; NA=not available
Discussion
In this large nationwide population based cohort study, where 45 232 children were exposed to antenatal corticosteroids, we found that after one course of antenatal corticosteroids, children had significantly higher cumulative incidence rates and hazards for overall serious infection (hazard ratios ranging from 1.24 to 1.38, P<0.001), pneumonia (1.22-1.39, P=0.05 to P<0.001), and acute gastroenteritis (1.35-1.52, P=0.01 to P<0.001) for the first three, six, and 12 months of life than children not exposed to antenatal corticosteroids. For sepsis (hazard ratios ranging from 1.44 to 1.74), the results were significant for the first six (P=0.01) and 12 (P=0.02) months of life. Associations were also found in the sibling matched analysis, indicating that unmeasured familiar confounding did not explain the observed associations and thus strengthening the reliability of our main findings. Our subgroup analyses indicated that the increased risk of serious infection after exposure to antenatal corticosteroids seemed to be more pronounced among children born at full term than preterm. Hence the results of our study provided real world evidence showing the associations between antenatal corticosteroids and serious infection in children, based on IPTW and sibling matched analyses, in a whole nation population over a study period of 12 years.
Clinical implications
Our findings have several implications. Firstly, although the immediate benefits of antenatal corticosteroids for reducing neonatal mortality and respiratory morbidities in preterm infants are well established, the subsequent latent risk of serious infection remains unknown and unnoticed. Our study adds real world evidence of the increased risk of rare but serious infection, especially overall serious infection, sepsis, pneumonia, and acute gastroenteritis, in children exposed to antenatal corticosteroids, indicating that antenatal corticosteroids might not be a wholly benign treatment. Secondly, our findings are consistent with previous studies that reported an increased risk of sepsis and congenital pneumonia in neonates exposed to antenatal corticosteroids.111213 The previous studies, however, did not look at shared factors within families and focused only on the immediate postnatal period. Our study highlights the association between antenatal corticosteroids and serious infection, including overall serious infection, sepsis, pneumonia, and acute gastroenteritis, in children beyond the neonatal period. Our results therefore suggest that children exposed to antenatal corticosteroids should be monitored carefully for serious infections. Clinicians might wish to weigh the benefits of antenatal corticosteroids in lowering the rates of neonatal respiratory distress syndrome (4.3%) and perinatal and neonatal mortalities (2.3-2.6%)24 against increasing the rates of overall serious infection during the first three (0.4%), six (0.9%), and 12 (1.7%) months of life reported in this study.
Thirdly, the increased risk of serious infection associated with exposure to antenatal corticosteroids seemed to be more pronounced in children born at full term versus preterm. These findings suggest that the benefits of antenatal corticosteroids for accelerating fetal lung maturation outweigh the potential risk of serious infections associated with antenatal corticosteroids in children born preterm, although the benefit-to-risk ratio might be reversed in children born at full term. In line with our findings, recent real world studies and meta-analyses reported a significantly higher risk of long term adverse neurocognitive or psychological outcomes in children born full term or late preterm, but not early preterm.91025 Although the well established immediate benefits of antenatal corticosteroids in children born before 34 weeks of gestation might potentially override the long term risks of adverse events, recent evidence suggests modest benefits of this treatment in children born late preterm or full term.23456 Because of the difficulty in predicting preterm birth, extending the use of antenatal corticosteroids beyond 34 weeks of gestation might lead to substantial increases in the number of children exposed to antenatal corticosteroids born at full term. This speculation is supported in our study, because about 40% of children exposed to antenatal corticosteroids were born at full term. Clinicians considering prescribing antenatal corticosteroids to pregnant individuals after 34 weeks of gestation should be aware of the risks of rare but serious infections in children. Further studies are needed to investigate the long term effects of antenatal corticosteroids beyond the neonatal period, particularly among children born at full term.
Plausible mechanisms
Several biological mechanisms could partly explain our findings. Corticosteroids could affect differentiation of lung epithelial and mesenchymal cells and induce production of surfactant,262728 but might be likely to pose a latent risk of serious infection in the first few months of life. Previous studies have reported that 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD2) converts innate corticosteroids to an inactive form and protects the fetus from high levels of maternal corticosteroids.2930 However, the affinity of 11β-HSD2 for synthetic corticosteroids, specifically betamethasone and dexamethasone, is low. These synthetic corticosteroids might pass through the placental barrier2930 and increase the risk of serious infection in children exposed to antenatal corticosteroids in the uterus. Corticosteroids have been known as anti-inflammatory agents modulating type 1 T helper (Th1)/Th2 responses. Priming of Th1/Th2 regulation related to exogenous corticosteroids occurring in late gestation might affect inflammatory conditions in children, particularly in the first few months of life. Further investigation to better understand the underlying biological mechanisms of our findings is warranted.
Strengths and limitations of this study
The main strengths of this study include the robust design and use of a nationwide database of 1 960 545 mother-child pairs over 12 years to investigate the associations between antenatal corticosteroids and risk of clinically important serious infection in children during the first few months of life. The universal single payer healthcare system and the corresponding research database in Taiwan allowed us to identify all mother-child pairs and prescriptions for antenatal corticosteroids, minimising potential selection bias and misclassification bias. The large sample size ensured adequate statistical power for the cohort and sibling matched analyses. Also, we conducted various algorithms, such as IPTW, sibling matched analyses, and sensitivity analyses to deal with systematic differences at baseline for confounding effects, familiar shared genetic and environmental factors, and robustness of the observed findings, respectively.
Our study had some limitations. Firstly, the population data were from one country (Taiwan) and therefore further investigation is needed to generalise or replicate the findings in other populations. Secondly, we could not include educational data and smoking status in the adjusted models because these data are not available in the National Health Insurance Research Database. Because we included socioeconomic status (which is correlated with education) and chronic obstructive pulmonary disease (which is correlated with smoking) in the models, their potential confounding effect could have been controlled for to a certain extent. Unmeasured residual confounding could remain, although we applied vigorous analytical approaches (eg, IPTW, robust variance estimator, and sibling matched analyses). Furthermore, although we controlled for differences in premature rupture of membranes, gestational diabetes, and gestational hypertension between pregnant individuals who received and did not receive antenatal corticosteroids by applying IPTW (a propensity score method), the differences between the two groups could still have biased our results. Nonetheless, the findings were similar when pregnant individuals with premature rupture of membranes, gestational diabetes, or gestational hypertension were excluded from the analysis. Thirdly, in common with all epidemiological studies, coding error is possible but differences in coding error between the exposed and non-exposed groups are unlikely. Also, we could not determine whether serious infections were caused by bacterial or viral infections because data related to laboratory tests for bacterial or viral infections are not available in the National Health Insurance Research Database.
Finally, confounding by indication should be considered because the offspring of pregnant individuals at risk of preterm delivery might already have an increased risk of serious infection. Similar to the findings in a previous study,10 the increased risk of adverse outcomes associated with exposure to antenatal corticosteroids seemed to be more pronounced among children born at full term than preterm, indicating that the influence of confounding by indication should be limited. Moreover, the uncertainty in these estimates might weaken the risk of serious infection noted in the study and therefore our results should be interpreted with caution.
Conclusion
In this nationwide population based cohort study, one course of antenatal corticosteroids was significantly associated with a 1.2-1.7-fold increased risk of serious infection, especially overall serious infection, sepsis, pneumonia, and acute gastroenteritis, in children during the first 12 months of life. Clinicians need to be aware of the increased risk of rare but serious infection among children exposed to antenatal corticosteroids.
What is already known on this topic
-
Numerous studies have reported the benefits of antenatal corticosteroids in reducing neonatal mortality and morbidities
-
Data on the potential harms of antenatal corticosteroids for serious infection in children are scarce, and rigorous evidence based on large population based cohorts is lacking
What this study adds
-
In this nationwide population based cohort study of 1 960 545 children born in Taiwan, one course of antenatal corticosteroids was significantly associated with a 1.2-1.7-fold increased risk of overall serious infection, sepsis, pneumonia, and acute gastroenteritis during the first 12 months of life
-
A similar risk of serious infection was observed in a sibling matched cohort
-
The potential long term risks of rare but serious infection associated with antenatal corticosteroids need to be weighed against their short term benefits in the perinatal period