Infection
Evolving viruses to fight bacterial infections
Multidrug-resistant bacterial infections are one of the most pressing issues in medicine, a situation that is only expected to worsen in the coming decades. The problem is being addressed not only by developing new antibiotics but also by studying antibiotic alternatives, such as phages. Among them is the Microbial Molecular Evolution Research Group at the Max Planck Institute for Evolutionary Biology in Plön.
Phages—viruses that can only infect bacteria—have been shown to fight some bacterial infections very effectively. Yet, little is known about the long-term success of most phage therapies. This includes knowledge of how bacteria can become resistant to phage treatment and, more importantly, whether and how phages can overcome such resistance.
In their recently published paper in Molecular Biology and Evolution, researchers from the Max Planck Institute for Evolutionary Biology show that Escherichia coli C can easily become resistant to phage ΦX174 by modifying its membrane surface molecules. The researchers show that this resistance can be overcome by ΦX174 under a particular evolutionary regimen. This research helps us to understand the evolution of bacterial resistance to phage infection, and demonstrates that—under the right conditions—phages can overcome this resistance.
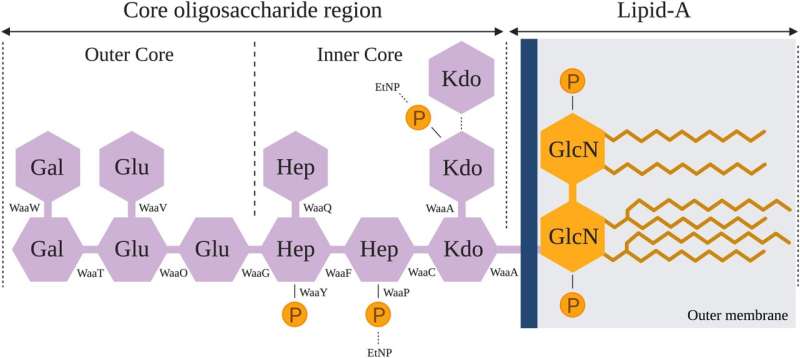
Bacteria quickly evolve resistance when they are faced with a threat, be it antibiotic or phage treatment. The nature of the threat determines the type of resistance bacteria that evolves. In a set of experiments, Romeyer Dherbey and colleagues show that the bacterium E. coli C becomes resistant to ΦX174 infection by modifying its outer membrane molecules—lipopolysaccharides (LPS).
Once the outer membrane of E. coli changes, the phages cannot attach to the membrane anymore and hence cannot infect the bacterium. Genome sequencing and phenotyping of the evolved bacteria showed that there is a great variety of evolved LPS variants that prevent infection by wildtype phage.
For phage therapeutics to be effective, bacterial phage resistance needs to be overcome. In contrast to antibiotics, where resistance is usually overcome by increasing antibiotic dose or changing the antibiotic, phages can themselves evolve to infect resistant bacteria. Dherbey and the team show that some types of resistance can be overcome over the course of very few phage generations (“easy” resistant bacterial strains). Other “hard” resistant strains could only be infected by recombining phages that evolved to infect easy resistant bacteria.
The experiments show that an experimental evolution framework could be a viable strategy to develop evolution-proof therapies in the future. Moreover, it may be possible to breed well-studied phage model systems to infect dangerous bacterial pathogens. If breeding effective phage populations is possible, then the time and cost-intensive steps of isolating safe and effective phages from the environment can be minimized.
More information:
Jordan Romeyer Dherbey et al, Stepwise Evolution of E. coli C and ΦX174 Reveals Unexpected Lipopolysaccharide (LPS) Diversity, Molecular Biology and Evolution (2023). DOI: 10.1093/molbev/msad154
Provided by
Max Planck Society
Citation:
Evolving viruses to fight bacterial infections (2023, August 2)
retrieved 2 August 2023
from https://phys.org/news/2023-08-evolving-viruses-bacterial-infections.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.