Cancer and neoplasms
Investigating pathogenic SNP of PKCι in HCV-induced hepatocellular carcinoma
Abstract
Hepatocellular carcinoma is a leading cause of cancer-related deaths due to its complexity in diagnosis, chemo-resistance, and aggressive nature. Identifying pathogenic single nucleotide polymorphism (SNP) in protein kinase C iota (PKCι) can be a potential biomarker in the prognosis and treatment of HCC. This study investigated the association between a SNP in PRKCI and the Pakistani population’s hepatocellular carcinoma (HCC) risk. Obtained samples were first evaluated for ALT measurements and viral load quantification through reverse transcriptase-PCR. The PKCι nsSNP rs1199520604 was evaluated computationally by multiple consensus bioinformatics tools for predicting its potential deleterious effects. Its association with hepatitis C virus- (HCV) mediated HCC was then investigated through ARMS-PCR (Amplification Refractory Mutation System Polymerase Chain Reaction). SNP analysis of rs1199520604 was performed in 100 cases and 100 controls. Variant rs1199520604’s homozygous T genotype is a risk factor allele for the HCV-induced HCC (odds ratio: 4.13, relative risk: 2.01, P-value < 0.0001). The heterozygous genotype is determined to protect HCV patients from HCC development (P < 0.001). The study highlighted the disease association of variant rs1199520604 with HCV-induced HCC in the Pakistani populations. This variant, after further validation through high-throughput investigation on a larger cohort, has the potential to be translated at the clinical level.
Introduction
Among cancer-related deaths, liver cancer is the second leading cause of worldwide deaths. Hepatocellular carcinoma (HCC) is the most frequently occurring type of liver cancer, especially in developing countries1 due to its complexity in diagnosis, chemo-resistance, and aggressive nature. It begins in hepatocytes and accounts for 75–85% of all liver cancer cases. Its occurrence rates change from 5.1 per 100,000 person-years in Europe to 17.7 per 100,000 in eastern Asia2. Pakistan alone accounted for 4354 new cases and 4365 deaths due to liver cancer in 2020. These disparate rates between regions highlight differences in the occurrence of risk factors3. In addition to other risk factors, one of the prominent factors associated with HCC development is Hepatitis C Virus (HCV)4.
Single nucleotide polymorphism (SNP) is a common genetic variation among individuals. SNPs have arisen as genetic markers for diseases, and numerous SNP markers are available in public databases. Previous studies have shown the importance of defining mutations as deleterious or non-deleterious and their association with certain diseases. SNPs in the genes GRIK1, MICA, HLA-DQA/DQB, and KIF1B have been reported to be associated with the risk of HCC5. The PKC family proteins have been positively associated with HCC in several studies6,7,8. However, those studies usually indicated its involvement in the carcinogenic process at a functional level. Any genetic association, such as the presence of SNPs in the PKCι coding gene (PRKCI) and HCC susceptibility and progression, is not yet reported.
PKCι is a lipid-dependent serine/threonine kinase. It participates in several signaling pathways that regulate cell survival9,10,11, differentiation10, polarity12, and microtubule dynamics in the early secretory pathway13. PKCι belongs to the evolutionarily conserved PKC family. It has 596 amino acids, and its molecular mass is 68,262 Da. PKCι activity is regulated by lipid second messengers (ceramide, phosphatidylinositol 3,4,5-triphosphate, and phosphatidic acid), phosphoinositide-dependent kinase (PDK1), tyrosine phosphorylation, and specific protein–protein interactions. Compared to other isozymes of PKC, PRKCI is more conserved from an evolutionary point of view. The overall amino acid sequence homology of PKCι and PKCζ is 72%, while the kinase domains are 86% identical. PKCι shows less homology with the other isoforms of PKC; for example, even in the highly conserved catalytic domain, it is only 53% identical with other PKC family proteins14. Polymorphisms in the PKC gene family have been previously associated with the occurrence of thyroid follicular neoplasms and fibrosarcoma. Non-synonymous SNPs in the PKC family have also been linked with fibroblast transformation. PRKCI genetic associations, such as the presence of SNPs in the coding gene and HCC susceptibility and progression, have yet to be reported. Considering the unique oncogenic potential of PRKCI and the gap in data regarding the role of its variants in HCC progression, we decided to investigate the association between a high-risk PRKCI variant rs1199520604 and HCV-induced HCC in the current study.
Materials and methods
SNP selection
The variants data was retrieved from ENSEMBL (ENSEMBL gene ID: ENSG00000163558). Missense variants were sorted and mapped on transcript ENST00000295797. Using ENSEMBL obtained data from different consensus tools (SIFT, PolyPhen2.0, MutationAssssor, CADD, REVEL, and MetaLR), the pathogenicity of the variants was predicted. The criteria for variant classification into disease-causing or tolerant class was chosen from the15 study. The variant predicted to be the most pathogenic was selected for further validation.
Primer designing
ARMS PCR (Amplification Refractory Mutation System Polymerase Chain Reaction) primers against the identified potential common damaging SNP rs1199520604 were designed using primer 1 software16,17. The chromosome assembly used as input in Primer1 was 38.p13.
Study population
One hundred samples from the HCC patients and 100 control samples were collected for SNP analysis. The ethical review board of ASAB’s parent institution, the National University of Sciences and Technology, approved the current study. Blood samples were taken at the Combined Military Hospital in Rawalpindi with verbal and written permission. The research was conducted per the Helsinki declaration’s standards and principles. Informed consent was obtained from all subjects and/or their legal guardian(s). Patients with co-morbidity with cardiac or metabolic diseases were excluded, and the sample was collected only from patients having HCV infection (Fig. 1). ALT levels were measured to assess liver function. Patients had significantly higher ALT levels than healthy controls (Result is provided in Fig. 2).
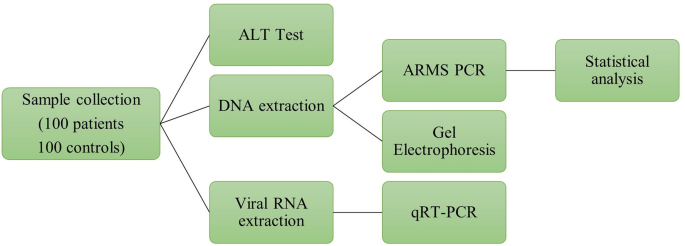
Schematic diagram of different in-vitro methods employed in this study. Samples were collected and processed for the ALT level test. Viral RNA was extracted from HCC patient samples, and qRT-PCR was performed on the extracted RNA. Lastly, genomic DNA was extracted from the samples. The extracted DNA was detected through gel-electrophoresis followed by performing ARMS-PCR and applying statistics on the results of ARMS-PCR.

Comparison of ALT concentration in HCC patients and control. ALT level is higher in HCC patients than control samples.
Genomic DNA extraction and SNP analysis
The method used for Genomic DNA extraction was the phenol–chloroform method8,18. ARMS-PCR was performed to detect single nucleotide change in PRKCI. For this type of PCR, specific tetra primers, two internal primers (forward and reverse) and two external primers (forward and reverse) against the selected SNP were used. The SNP-specific primers were inner primers that were used for detection. The sequences for primers are: Forward inner 5′-GTGAAAGCCTACTACCACG-3′, Reverse inner 5′-TCCCAGGACACTCATCA-3′, Outer forward 5′-AGGTGGGCAGGTAGGT-3′, and Outer reverse 5′-CACCCCTATCACTTCGTC-3′. PCR products were run on 2% gel, and band size was determined by comparing it with Thermo Scientific GeneRuler 100 bp DNA Ladder.
Viral RNA extraction
For the analysis of the viral load in the HCC patients, viral RNA was extracted from the patient’s blood via FAVOREGEN KIT®. For this purpose, 200 µl of serum was combined in a 1.5 mL microcentrifuge tube with 500 µl of VNE buffer. This mixture was vortexed for 5–7 s. 500 µl of 75% ethanol was added and vortexed again for 5–7 s. It was then poured into a spin column and centrifuged at 8000 rpm for 1 min. The spin column was then removed, and the collection tube was discarded. RNA was retained on the filter of the spin column, which was then placed in a new collection tube, and 500 µl of wash buffer 1 was added into the spin column and centrifuged at 8000 rpm for 1 min. The collecting tube was changed, and 750 µl of wash buffer 2 was added and centrifuged at 14,000 rpm for 1 min. The centrifugation step was repeated twice to dry the filter. Lastly, the filter was placed in a new 1.5 mL microcentrifuge tube, and 50 µl of RNase-free water was added. The Eppendorf with the filter was centrifuged at 8000 rpm for 1 min. The RNA eluate was stored at −20 °C.
Quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) to determine viral load
Blood viral RNA was extracted using the FavorPrepTM Viral DNA/RNA Kit to evaluate the viral load in a patient sample (catalog no. FAVNK 001–1). 200 µl of Blood and 500 µl of VEN buffer were combined in an Eppendorf tube for 5–7 s vortexing. The tube was vortexed for at least 5–7 s after adding 500 µl of 75% ethanol. The material was transported to the spin column and spun for one minute at 8000 rpm. The collecting tube was discarded, and a fresh tube was inserted with the filter tube. After 500 µl of wash buffer 1 was added and centrifuged, the mixture was centrifuged for 1 min at 8000 rpm. The collecting tube was replaced, 750 µl of wash buffer 2 was added, and the tube was centrifuged for one minute at 14,000 rpm. This approach was used twice to dry the filter. Transferred filter tube to Eppendorf tube, added 50 µl of RNase-free water and centrifuged at 8000 rpm for another minute. After transferring the RNA sample to a tube, it was stored at −20 °C.
Statistical analysis
The collected genotyping data were analyzed statistically using GraphPad Prism 9. The Chi-square test was performed on both the patient and control groups. Furthermore, risk and odds ratios and calculated confidence intervals were measured through Fisher Exact test. Statistical significance was assumed when the p-value was less than 0.005.
Ethics approval
Approval for the study was obtained from the Institutional Review Board of National University of Science and Technology (NUST), Pakistan (IRB No. 10-2021-01/01). Informed consent was obtained from all subjects and/or their legal guardian(s).
Results
Association of PKCι SNP (rs1199520604) with HCV-mediated HCC
Multiple consensus tools revealed variant rs1199520604 pathogenicity; therefore, this variant was further validated for its association with HCV-mediated HCC. Association was studied in 100 patients and 100 healthy individuals through genotyping PCR. The analysis revealed a significant association of the SNP in homozygous mutated form (i.e., TT) with HCC compared to the heterozygous GT genotype and homozygous wild genotype GG (Table 1; odds ratio 1.134, relative risk 2.012, P-value < 0.0001). This shows that the polymorphism with the TT genotype increased the risk of disease occurrence.
Gender based association of PKCι SNP (rs1199520604) with HCV-mediated HCC
A comparison of PRKCI polymorphism with HCC patients and controls was performed (Table 2). The data obtained support the results described in Table 1. In both males and females, the mutated homozygous allele TT was found to be associated with the disease. The P-value for males and females having allele TT was 0.0006 and 0.0015, respectively, emphasizing the significance of the results.
Relationship of PKCι SNP rs1199520604 alleles with viral load in HCV induced HCC patients
Viral titer in patients was measured by qRT-PCR. The analysis of viral load against PKCι SNP rs1199520604 alleles shows significant differences. Average viral copy number for alleles GG, TT, and GT of rs1199520604 were determined and then analyzed, which shows that patients with GG allele have higher (560,095,306.7 copies/ml) viral copy number compared to patients with TT and GT alleles, which suggests that there may be a correlation of viral load and rs1199520604 genotypes (Fig. 3).

HCV Viral load plotted against homozygous wild (GG), heterozygous (GT) and homozygous mutated (TT) genotypes of HCC patients. GG allele has significantly high viral load compared to TT and GT alleles.
Discussion
Several genetic and environmental factors are involved in causing hepatocellular carcinoma (HCC). In addition to other risk factors, one of the prominent factors associated with HCC progression is the hepatitis C virus (HCV)4. HCV-induced HCC occurrence is increasing globally, particularly in developing countries. A significant problem with most cancers is detecting the disease in its early stages, as is the case for HCC. The change in protein expression and its structural and functional properties have often been associated with the presence of certain kinds of polymorphisms in the genetic sequence19,20,21; however, not much is known about the role of PRKCI variations about natural polymorphism. It is, therefore, crucial to identify deleterious nsSNPs in the PRKCI gene, as nsSNPs cause the most significant damaging impact on the protein structure and function22.
The current study only considered missense variants because of their direct involvement in disease pathologies and their impacts on the adopted treatment regimen. So, in this study, SNP in PRKCI was correlated with HCC to identify a new prognostic marker for HCC. The selected SNP (rs1199520604), when processed through different computational tools, was the most deleterious. This SNP (rs1199520604) falls within the PB1 domain (which is exclusive to atypical PKCs) of PKCι23. The SNP changes the amino acid Glycine to Tryptophan at position 34. The PB1 domain facilitates protein–protein interactions between PKCι and other proteins having PB1 domain, such as Par-6 (partitioning-defective 6)12,24, ZIP/p6225, and MEK5 (MAPK (mitogen-activated protein kinase)/ERK (extracellular-signal-regulated kinase) kinase 5)26. The PB1 domain is near a highly conserved region, making it a striking therapeutic target for cancer treatment23. The analysis of PCR results after amplifying the SNP in 200 samples (100 controls and 100 HCV-induced HCC patients) revealed that the homozygous mutant allele TT had a significant correlation with HCC compared to homozygous GG and heterozygous GT alleles. The analysis of PCR data concerning gender also indicated the significant association of the same alleles in both males and females with the disease. However, the relative risk and odds ratio in males was slightly more significant than in females. This difference could also be notable in the higher incidence rate of HCC in males than in females27,28. The association of polymorphism with genetic disease gives us an idea about susceptibility and can also be used for early diagnosis29,30. Thus, this SNP (rs1199520604) could be a potential biomarker for the prognosis of HCC.
It has been established that high alanine aminotransferase level is linked with HCV-induced HCC and can lead to rapid disease development31. The ALT levels in HCC patients compared to control revealed significantly increased ALT levels in HCC patients (106 IU/L). The association of viral load with rs1199520604 alleles was performed to analyze the link of genotype with viral load. Our results demonstrated that patients with genotype GG have a high viral load, followed by TT and GT. It might be due to clearance of viral load over time as literature shows clearance of HCV viral RNA in patients coinfected with HCV/HIV-1 having rs12979860 polymorphism CC genotype32.
Conclusion
In conclusion, our study demonstrated that the pathogenic SNP (rs1199520604) of PKCι, identified through computational tools, is strongly associated with HCV-induced HCC. This association indicates that the SNP (rs1199520604) may serve as a prognostic marker for HCV-induced HCC. High ALT levels were observed in HCC patients, and a correlation between PKCι SNP rs1199520604 genotypes and viral loads gave insight that there may be an association between them. The expression profile of PKCι upon G34W mutation needs to be further explored to elucidate its role as a prognostic or diagnostic marker and open new therapeutic avenues for HCC treatment. The association between the viral load and PKCι SNP rs1199520604 genotypes need to be studied at the molecular level to explain the correlation better. Further in vitro and in vivo studies are required to ascertain the effects of the variant on native protein structure and function and how tumor progression is affected.
Data availability
All the relevant data has been provided in the manuscript used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
-
Srivatanakul, P., Sriplung, H. & Deerasamee, S. J. A. P. J. O. C. P. Epidemiology of liver cancer: An overview. Asian Pac. J. Cancer Prevent. 5, 118–125 (2004).
-
Ferlay, J. et al. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur. J. Cancer 103, 356–387 (2018).
Google Scholar
-
Kulik, L. & El-Serag, H. B. J. G. Epidemiology and management of hepatocellular carcinoma. Gastroenterology 156, 477-491. E471 (2019).
Google Scholar
-
Vescovo, T. et al. Molecular mechanisms of hepatitis C virus–induced hepatocellular carcinoma. Clin. Microbiol. Infect. 22, 853–861 (2016).
Google Scholar
-
Chen, Z. et al. ACYP2 polymorphisms are associated with the risk of liver cancer in a Han Chinese population. Oncotarget 8, 67723–67731. https://doi.org/10.18632/oncotarget.18574 (2017).
Google Scholar
-
Mandal, J. P. et al. PKCδ mediates mitochondrial ROS generation and oxidation of HSP60 to relieve RKIP inhibition on MAPK pathway for HCC progression. Free Radical Biol. Med. 163, 69–87 (2021).
Google Scholar
-
Du, G.-S. et al. Expression of P-aPKC-ι, E-cadherin, and β-catenin related to invasion and metastasis in hepatocellular carcinoma. Ann. Surg. Oncol. 16, 1578–1586 (2009).
Google Scholar
-
Shahid, K. et al. Pathogenicity of PKCγ genetic variants—possible function as a non-invasive diagnostic biomarker in ovarian cancer. Genes 14, 236 (2023).
Google Scholar
-
Sanz, L., Sanchez, P., Lallena, M. J., Diaz-Meco, M. T. & Moscat, J. J. T. E. J. The interaction of p62 with RIP links the atypical PKCs to NF-κB activation. EMBO J. 18, 3044–3053 (1999).
Google Scholar
-
Wooten, M. W., Seibenhener, M. L., Neidigh, K. B. & Vandenplas, M. L. J. M. Mapping of atypical protein kinase C within the nerve growth factor signaling cascade: Relationship to differentiation and survival of PC12 cells. Mol. Cell. Biol. 20, 4494–4504 (2000).
Google Scholar
-
Xie, J., Guo, Q., Zhu, H., Wooten, M. W. & Mattson, M. P. J. M. B. R. Protein kinase C iota protects neural cells against apoptosis induced by amyloid β-peptide. Mol. Brain Res. 82, 107–113 (2000).
Google Scholar
-
Joberty, G., Petersen, C., Gao, L. & Macara, I. G. J. N. C. B. The cell-polarity protein Par6 links Par3 and atypical protein kinase C to Cdc42. Nat. Cell biol. 2, 531–539 (2000).
Google Scholar
-
Tisdale, M. J. J. N. R. C. Cachexia in cancer patients. Nat. Rev. 2, 862–871 (2002).
Google Scholar
-
Selbie, L. A., Schmitz-Peiffer, C., Sheng, Y. & Biden, T. J. J. J. O. B. C. Molecular cloning and characterization of PKC iota, an atypical isoform of protein kinase C derived from insulin-secreting cells. J. Biol. Chem. 268, 24296–24302 (1993).
Google Scholar
-
Khan, K. et al. Influence of PRKCE non-synonymous variants on protein dynamics and functionality. Hum. Mol. Genet. 31, 2236 (2022).
Google Scholar
-
Collins, A. & Ke, X. J. T. O. B. J. Primer1: Primer design web service for tetra-primer ARMS-PCR. Open Bioinform. J. 6, 55 (2012).
Google Scholar
-
Newton, A. C. J. C. R. Protein kinase C: Structural and spatial regulation by phosphorylation, cofactors, and macromolecular interactions. Chem. Rev. 101, 2353–2364 (2001).
Google Scholar
-
Shabbir, M. et al. Association of CTLA-4 and IL-4 polymorphisms in viral induced liver cancer. BMC Cancer 22, 1–9 (2022).
Google Scholar
-
De Cock, V. C. et al. Restoration of normal motor control in Parkinson’s disease during REM sleep. Brain 130, 450–456 (2007).
Google Scholar
-
Fu, S., Fedota, J. R., Greenwood, P. M. & Parasuraman, R. J. B. P. Dissociation of visual C1 and P1 components as a function of attentional load: an event-related potential study. Biol. Psychol. 85, 171–178 (2010).
Google Scholar
-
Robert, F. & Pelletier, J. J. F. I. G. Exploring the impact of single-nucleotide polymorphisms on translation. Front. Genet. 9, 507 (2018).
Google Scholar
-
Yazar, M. & Özbek, P. J. O. A. J. O. I. B. In silico tools and approaches for the prediction of functional and structural effects of single-nucleotide polymorphisms on proteins: An expert review. OMICS: A J. Integr. Biol. 25, 23–37 (2021).
Google Scholar
-
Parker, P. J., Justilien, V., Riou, P., Linch, M. & Fields, A. P. J. B. P. Atypical protein kinase Cι as a human oncogene and therapeutic target. Biochem. Pharmacol. 88, 1–11 (2014).
Google Scholar
-
Qiu, R.-G., Abo, A. & Martin, G. S. J. C. B. A human homolog of the C. elegans polarity determinant Par-6 links Rac and Cdc42 to PKCζ signaling and cell transformation. Curr. Biol. 10, 697–707 (2000).
Google Scholar
-
Puls, A., Schmidt, S., Grawe, F. & Stabel, S. J. P. O. t. N. A. O. S. Interaction of protein kinase C ζ with ZIP, a novel protein kinase C-binding protein. 94, 6191–6196 (1997).
-
Wooten, M. W. et al. The atypical protein kinase C-interacting protein p62 is a scaffold for NF-κB activation by nerve growth factor. J. Biol. Chem. 276, 7709–7712 (2001).
Google Scholar
-
Hafeez, S., Mahmood, A., Khan, R. U. & Malkani, N. J. J. O. B. M. Trends in cancer prevalence in Punjab, Pakistan: A systematic study from 2010 to 2016. J. Bioresource Manag. 7, 8 (2020).
Google Scholar
-
Singal, A. G., Lampertico, P. & Nahon, P. J. J. O. H. Epidemiology and surveillance for hepatocellular carcinoma: New trends. J. Hepatol. 72, 250–261 (2020).
Google Scholar
-
Kamaraj, B. & Bogaerts, A. J. P. O. Structure and function of p53-DNA complexes with inactivation and rescue mutations: A molecular dynamics simulation study. PLoS ONE 10, 4638 (2015).
Google Scholar
-
Kamaraj, B. & Purohit, R. J. B. R. I. In silico screening and molecular dynamics simulation of disease-associated nsSNP in TYRP1 gene and its structural consequences in OCA3. 2013 (2013).
-
Tarao, K. et al. Association between high serum alanine aminotransferase levels and more rapid development and higher rate of incidence of hepatocellular carcinoma in patients with hepatitis C virus-associated cirrhosis. Cancer 86, 589–595 (1999).
Google Scholar
-
Lapiński, T. W., Pogorzelska, J., Kowalczuk, O., Nikliński, J. & Flisiak, R. J. P. E. SNP RS12979860 related spontaneous clearance of hepatitis c virus infection in HCV/HIV-1 coinfected patients. Przegl. Epidemiol. 67, 407–409 (2013).
Google Scholar
Acknowledgements
The authors extended their appreciation to the Researchers Supporting project number (RSP2023R502), as King Saud University, Riyadh Saudi Arabia, for funding this project.
Funding
The project was funded by the Researcher supporting project number (RSP2023R502), King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
Conceptualization, N.K, KK., Y.B, N.M.A and S.R.; methodology, M.S., LD, N.K, K.K., and N.M.A.; experimentation, N.K., K.K.; validation .S.R; formal analysis, NK., ZH, K.K, T.A., A.A., J.H.T. and L.D.; investigation, N.K., J.HT., K.K. and TA.; resources, M.S., S.R., ; data curation, N.M.A. SJ, S.R., ; writing—original draft preparation, J.H.T.; writing—review and editing, K.K., S.R, T.A, J.H.T., SJ, Y.B. and M.S.; visualization, N.K., A.A., M.S. and N.M.A.; supervision, M.S.; project administration, M.S.; funding acquisition, S.R., ZH, J.H.T. and M.S. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
Reprints and Permissions
About this article
Cite this article
Khan, N., Khan, K., Badshah, Y. et al. Investigating pathogenic SNP of PKCι in HCV-induced hepatocellular carcinoma.
Sci Rep 13, 12504 (2023). https://doi.org/10.1038/s41598-023-39804-0
-
Received: 02 February 2023
-
Accepted: 31 July 2023
-
Published: 02 August 2023
-
DOI: https://doi.org/10.1038/s41598-023-39804-0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.