Cancer and neoplasms
A Look at This Year’s MPN Updates
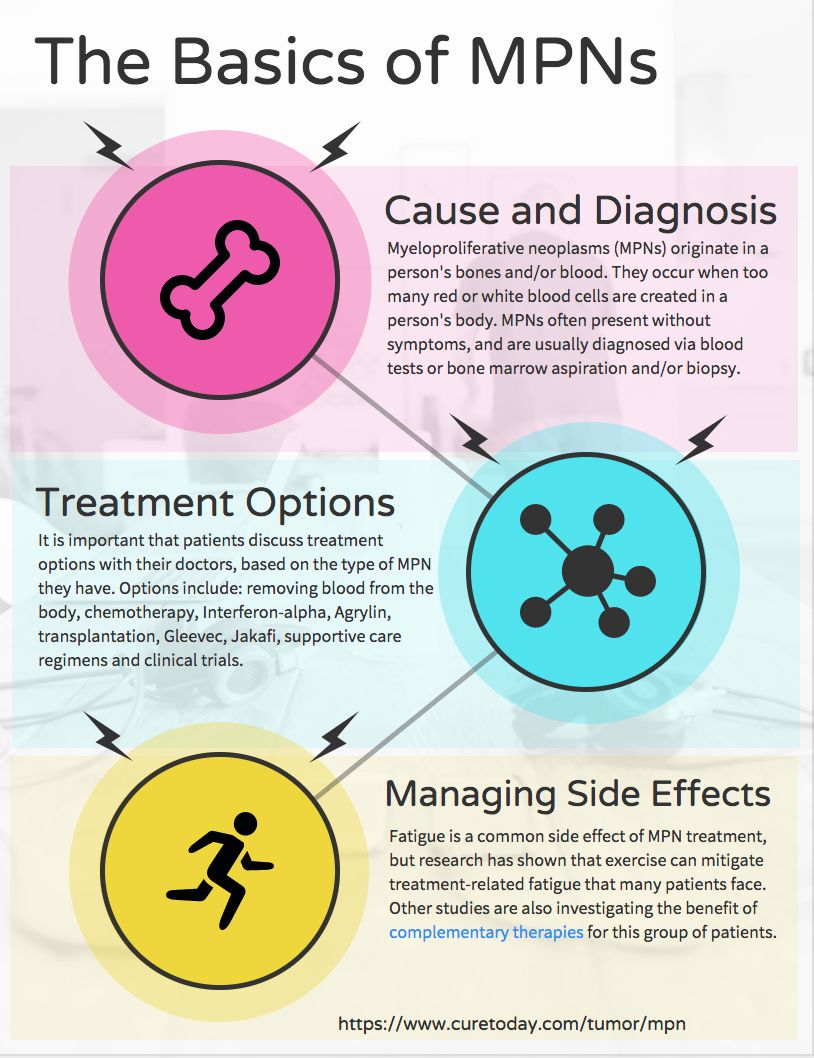
Myeloproliferative neoplasms (MPNs) are a cancer that occurs when the bone marrow makes too many red or white blood cells or platelets. While the group of cancers is rare, researchers, clinicians and patient advocates are continuously making strides for blood diseases — leading to better outcomes and quality of life for patients who have them.
CURE® looked back at some of the most insightful or exciting MPN content from 2023 so far. Here’s what we found:
One Step Closer to an MPN Cure
In our 2023 MPN Special Issue, experts discussed the promise of a type of drug called JAK inhibitors, which are helping patients with MPNs to live longer. However, there is still no cure.
“Patients with myeloproliferative neoplasms all have one unified theme of hyperactivity of the JAK-STAT signaling pathway that seems to be occurring in their bone marrow cells, and this is irrespective of the driver mutation present in these cells,” said Dr. John Mascarenhas, a professor of medicine at Icahn School of Medicine at Mount Sinai in New York who has also worked on multiple JAK inhibitor trials.
‘No Symptom Is Too Small’ for Patients With MPNS
Patients with MPNs may go through long periods where they do not experience any symptoms from their disease, but once they do, it is important to communicate these experiences with their health care team, Alfa Lafleur, an advanced practice registered nurse at Florida Cancer Specialists and Research Institute Trinity Cancer Center, explained in an interview with CURE®.
“My best advice to patients is to remind them that they are not alone in this disease. No symptom is too small to bring to the attention of your nurse who is more than willing to assist with the physical and mental challenges that come,” Lafleur said.
Besremi Designated as Preferred Treatment for Polycythemia Vera
In May, the National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology were updated to state that Besremi was the newly recommended therapy for patients with high-risk and low-risk polycythemia vera, regardless of their prior treatment history.
“This recent update to treatment guidelines by NCCN represents the community’s recognition of the value of Besremi as a therapeutic option for all adults with PV, regardless of their treatment history,” said Dr. Raymond Urbanski, head of clinical development and medical affairs for PharmaEssenta (the manufacturer of Besremi), in a press release. “Given its deep, durable control over the disease beyond the symptoms, we’re continuing to study Besremi in PV, as well as other MPNs and hematologic malignancies.”
FDA Grants Fast Track Designation for Xpovio for Myelofibrosis
This summer, the Food and Drug Administration (FDA) granted a fast track designation to Xpovio (Selinexor) for the treatment of primary myelofibrosis, post-essential thrombocythemia and post-polycythemia very myelofibrosis. By granting the designation, the FDA is agreed to speed up their review of the drug, which showed promise in treating this patient population in the XPORT-MF-034 clinical trial.
Trial results showed Xpovio’s promise in reducing spleen volume (enlarged spleen is a common complication of MPNs) and symptoms. Researchers are also investigating the drug’s effect on survival, anemia and other side effects.
Jakafi Improves Symptoms, Spleen Volume in Myelofibrosis
Results from the phase 3 COMFORT-I and COMFORT-II trials presented at the 2023 European Hematology Association Congress, showed that Jakafi (ruxolitinib) improved spleen volume and overall total symptom score, regardless of transfusion anemia or transfusion status in patients with myelofibrosis.
More specifically, study results showed that the reduction in spleen volume of 35% or greater from baseline rates at week 24 in patients with new or worsening anemia up to week 12 were 48.8%, 33.3% and 41.4%, respectively, for those who were nonanemic, anemic/nontransfusion dependent, and anemic/transfusion dependent at baseline. These rates were 43.2%, 23.1% and 28.2%, respectively, in patients who did not have new or worsening anemia at week 24.
For more news on cancer updates, research and education, don’t forget to subscribe to CURE®’s newsletters here.