Cancer and neoplasms
Potential factors for and the prognostic impact of ascites after allogeneic hematopoietic stem cell transplantation
Abstract
Ascites is sometimes detected after allogeneic hematopoietic stem cell transplantation (allo-HSCT); however, since limited information is currently available, its clinical meaning remains unclear. Therefore, we herein examined potential factors for and the impact of ascites on the prognosis of patients after allo-HSCT at our institutes. Fifty-eight patients developed ascites within 90 days of allo-HSCT (small in 34 (16%), moderate-large in 24 (11%)). A multivariate analysis identified veno-occlusive disease/sinusoidal obstruction syndrome (p = 0.01) and myeloablative conditioning (p = 0.01) as significant potential factors for the development of small ascites. Thrombotic microangiopathy (TMA) (p < 0.01) was a significant potential factor for moderate-large ascites. The incidence of both small and moderate-large ascites correlated with lower overall survival (p = 0.03 for small ascites and p < 0.01 for moderate-large ascites) and higher non-relapse mortality rates (p = 0.03 for small ascites and p < 0.01 for moderate-large ascites). Lower OS and higher NRM rates correlated with the incidence of both small and moderate-large ascites. Further investigation is warranted to establish whether the clinical sign of ascites improves the diagnostic quality of TMA in a large-scale study.
Introduction
Refractory and relapsed hematological malignancies generally have a poor prognosis. Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is a well-established curative approach for these diseases. Conditioning regimens before allo-HSCT eliminate residual malignant cells, and allo-HSCT, which involves the replacement of a recipient’s bone marrow with donor hematopoietic stem cells, induces the graft-versus-leukemia/lymphoma effect to minimal residual disease. Although allo-HSCT achieves the highest rate of a complete recovery, the non-relapse mortality (NRM) rate remains high. The non-infectious complications of allo-HSCT include graft-versus-host disease (GVHD), thrombotic microangiopathy (TMA), veno-occlusive disease/sinusoidal obstruction syndrome (VOD/SOS), and organ failure caused by conditioning chemotherapy and GVHD prophylaxis. Ascites is sometimes detected after allo-HSCT. Representative complications showing the clinical sign of ascites after allo-HSCT are VOD/SOS and acute or chronic GVHD1,2. The clinical sign of ascites has been incorporated into the diagnostic criteria for VOD/SOS3,4,5. However, we frequently encounter cases of ascites without these complications. Limited information is currently available on the development of ascites after allo-HSCT, including its frequency, potential factors, and impact on the prognosis of patients.
Therefore, a double-center collaborative retrospective study was herein performed on the development of ascites after allo-HSCT in 210 patients at our institutes to investigate its frequency, potential factors, and impact on patient prognosis.
Methods
Patients
Between 2000 and 2021, 260 consecutive patients were retrospectively recruited after first allo-HSCT at our institutes (Faculty of Medicine, Kagawa University and Takamatsu Red Cross Hospital, Kagawa, Japan). Fifty patients were excluded due to a lack of data on ascites (48/260 patients, 18%) and primary disease (2/260, 0.8%; multiple myeloma). Therefore, 210 patients were ultimately examined. The Institutional Review Boards of the Faculty of Medicine, Kagawa University and Takamatsu Red Cross Hospital approved the present study.
Abdominal computed tomography (CT) and abdominal ultrasound findings after allo-HSCT were reviewed. These imaging modalities were employed to identify the cause of clinical symptoms, including infections, elevated C-reactive protein levels, and organ dysfunction. Eligible patients were those in whom ascites was detected by a radiologist or abdominal ultrasound technician and confirmed by more than two physician researchers. In the present study, ascites was stratified into small and moderate-large, and defined as that in the rectovesical pouch and out of the rectovesical pouch by CT or abdominal ultrasound, respectively. Ascites limited to the rectovesical pouch was defined as small ascites, while that both in and out of the rectovesical pouch or only out of the rectovesical pouch was defined as moderate-large ascites.
Transplantation procedures
The classification of conditioning regimens by the Reduced-Intensity Conditioning Regimen workshop by the Center for International Blood and Marrow Transplant Research as myeloablative (MAC) or reduced intensity (RIC) was used in the present study6,7. GVHD prophylaxis was performed by the administration of the calcineurin inhibitors (CNI), cyclosporine and tacrolimus alone or in combination with mycophenolate mofetil (MMF) or short-term methotrexate (stMTX, 10-7-7 or 10-7-7-7 mg/m2). Anti-thymocyte globulin (ATG) was also administered to a subset of patients.
Definitions
The previously reported definition for the refined disease risk index (DRI) was employed in the present study8. An absolute neutrophil count of 0.5 × 109/L or higher on three consecutive days confirmed engraftment. Acute GVHD was diagnosed and graded according to the modified Glucksberg-Seattle criteria9,10. VOD/SOS and TMA were diagnosed according to the Seattle3, Baltimore4, EBMT5, BMT-CTN11, or IWG-EBMT criteria12. The number of days from transplantation to the confirmation of infection was used as the onset day of viral infection. The detection of 3 cytomegalovirus (CMV)-positive cells per 2 slides using the C10/C11 antigenemia assay or 2 CMV-positive cells per 50,000 white blood cells using the HRP-C7 antigenemia assay confirmed the reactivation of CMV.
Statistical analysis
In the present study, the 3-year overall survival (OS) rate was used as the primary endpoint, while 3-year cumulative incidence of relapse (CIR) and NRM rates and the relationships between patient characteristics and ascites after allo-HSCT were the secondary endpoints. OS was defined as the time from the landmark point (described below) until death from any cause. Morphological and/or imaging findings were used to diagnose disease relapse/progression. CIR and NRM were defined as the first documented relapse or progression and death without evidence of relapse or progression, respectively. The Kaplan–Meier method was employed to estimate OS probabilities, which were then compared using the Log-rank test. CIR and NRM probabilities were examined using cumulative incidence curves adjusted for competing risks and then compared with Gray’s method. The Cox proportional hazard regression model was employed to identify the factors influencing OS, and the Fine-Gray competing risk regression model for CIR and NRM. Univariate and multivariate analyses were performed to identify potential factors for ascites after allo-HSCT.
Regarding each potential factor, the univariate analysis compared the frequency of categorical or continuous variables between patients with and without ascites. Variables with a P value < 0.1 in the univariate analysis were then examined in the multivariate analysis and included age at allo-HSCT (younger than 50 vs. 50 years or older), sex (male vs. female), serum albumin levels before allo-HSCT (continuous variable), the primary disease (myeloid neoplasms vs. lymphoid and ambiguous lineage neoplasms), donor type (human leukocyte antigen [HLA]-matched related donor [MRD]/HLA-matched unrelated donor [MUD] vs. HLA-mismatched related donor [MMRD]/HLA-mismatched unrelated donor [MMUD] vs. cord blood [CB] vs. haploidentical), the use of ATG, the type of GVHD prophylaxis (CNI plus MTX vs. others), the type of conditioning regimen (MAC vs. RIC), the hematopoietic cell transplant-comorbidity index (0 vs. ≥ 1), neutrophil engraftment (< day 18 vs. ≥ day 18), and the occurrence of G2-4 acute GVHD (No vs. Yes), VOD/SOS (No vs. Yes), TMA (No vs. Yes), CMV viremia (No vs. Yes), and disease recurrence (No vs. Yes). The effects of neutrophil engraftment, G2-4 acute GVHD, VOD/SOS, TMA, CMV viremia, and disease recurrence on the development of ascites and clinical outcomes were investigated in patients who survived for 90 days after allo-HSCT. This landmark method excluded any potential bias caused by the inclusion of patients who died before these clinical events manifested in the group without these clinical events13,14.
Statistical analyses were all two-sided and P-values < 0.05 indicated a significant difference. Statistical analyses were conducted using EZR (version 1.33) (Saitama Medical Center, Jichi Medical University)15.
Ethical approval
The study complied with the declaration of Helsinki. This study was approved by the Institutional Review Boards of the Faculty of Medicine, Kagawa University and Takamatsu Red Cross Hospital.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Results
Patient characteristics
Table 1 shows the characteristics of patients who were retrospectively recruited in the present study. The median age of patients at allo-HSCT was 52 years (range, 16–74), and 113 (54%) were 50 years or older. There were 119 male patients (57%). The median serum albumin level before allo-HSCT was 4.0 g/dL (range, 2.7–5.1). Primary diseases were as follows: myeloid neoplasms in 128 (61%: acute myeloid leukemia in 84, myelodysplastic syndrome in 35, myeloproliferative neoplasms in 4, and chronic myelogenous leukemia in 5) and lymphoid and ambiguous lineage neoplasms in 82 (39%: precursor or ambiguous lineage neoplasms in 53 and mature neoplasms in 29). Refined DRI were low, intermediate, high, and very high in 16 (8%), 85 (40%), 72 (34%), and 37 (18%) patients, respectively. Donor types were MRD, MMRD, MUD, MMUD, CB, and haploidentical in 39 (19%), 5 (2%), 77 (37%), 56 (27%), 28 (13%), and 5 (2%) patients, respectively. GVHD prophylaxis involved CNI plus MTX in 182 patients (87%) and others in 28 (13%), while ATG was administered to a subset of 53 patients (25%). Regarding conditioning regimens, 147 patients received MAC (70%), while 63 were treated with RIC (30%). The median follow-up period among surviving patients was 44 months (range, 1–209).
Potential factors for ascites
Ascites was detected in 58 patients within 90 days of allo-HSCT (small in 34 (16%), moderate-large in 24 (11%)). None of the patients in the present cohort underwent surgical interventions. The univariate analysis identified late neutrophil engraftment (HR, 2.65; 95% CI 1.13–6.20; p = 0.03), G2-4 acute GVHD (HR, 2.36; 95% CI 1.03–5.39; p = 0.04), and VOD/SOS (HR, 5.82; 95% CI 1.72–19.70; p < 0.01) as significant potential factors for small ascites. RIC (HR, 0.27; 95% CI 0.08–0.95; p = 0.04) was also identified as a potential factor (Table 2). In the multivariate analysis, VOD/SOS (HR, 7.19; 95% CI 1.52–34.00; p = 0.01) and RIC (HR, 0.13; 95% CI 0.03–0.66; p = 0.01) remained as significant potential factors associated with small ascites.
We then investigated the development of moderate-large ascites using the same analyses. The univariate analysis identified TMA (HR, 27.50; 95% CI 7.12–106.00; p < 0.01) as a significant potential factor (Table 3), and this factor remained significant in the multivariate analysis (HR, 25.30; 95% CI 6.13–104.00; p < 0.01).
Serum albumin levels were not associated with the occurrence of small (p = 0.34) or moderate-large (p = 0.16) ascites in the univariate analysis.
Clinical diagnoses of ascites (n = 58) designated by the primary physician in the present cohort included infection in 6 patients (10%: small ascites in 5, moderate-large ascites in 1), GVHD in 17 (29%: small in 13, moderate-large in 4), VOD/SOS in 6 (10%: small in 4, moderate-large in 2), TMA in 12 (21%: small in 3, moderate-large in 9), and others in 17 (29%: small in 9, moderate-large in 8). Infections were treated by antibiotics (n = 6), GVHD by increasing immunosuppression using corticosteroids (n = 15) or MMF (n = 2), VOD/SOS using fresh frozen plasma (n = 6), and TMA by dose reductions in or discontinuing CNI (n = 10), fresh frozen plasma (n = 5), and recombinant thrombomodulin (n = 3) (the total number of treatment procedures). In cases in which it was possible to assess treatment effects, response rates for ascites were 75% (3 out of 4 patients) in infection cases, 73% (8 out of 11) in GVHD cases, 60% (3 out of 5) in VOD/SOS cases, and 75% (6 out of 8) in TMA cases. Furthermore, the attenuation of ascites was noted in 6 out of 8 patients with a good clinical response to the TMA treatment, in contrast to none of the patients with a poor clinical response.
Transplant outcomes
OS, CIR, and NRM
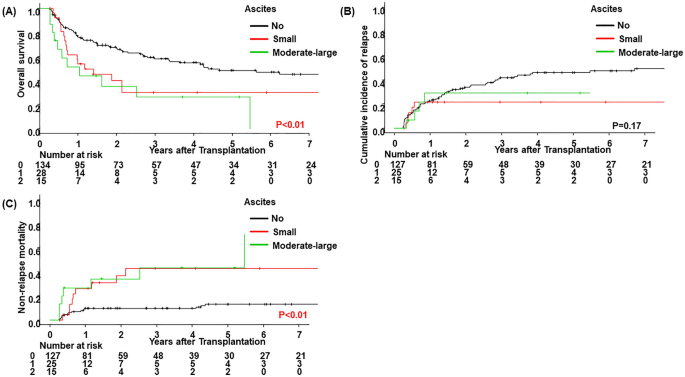
Three-year OS, CIR, and NRM rates in the present cohort were 51% (95% CI 43–59), 38% (95% CI 30–46), and 17% (95% CI 12–24), respectively. Following the stratification of ascites 90 days after allo-HSCT (no vs. small vs. moderate-large), a significantly lower OS rate was noted in patients with small (p = 0.03) and moderate-large ascites (p < 0.01). No significant differences were observed in the CIR rate in patients with small ascites (p = 0.10), whereas a significantly higher NRM rate was noted in patients with small (p < 0.01) and moderate-large ascites (p < 0.01) (Fig. 1, Table 4). In the multivariate analysis, the incidence of both small and moderate-large ascites correlated with a lower OS rate (HR, 1.85; 95% CI 1.06–3.21; p = 0.03 for small ascites and HR, 2.56; 95% CI 1.30–5.04; p < 0.01 for moderate-large ascites) (Table 5). The incidence of small ascites did not correlate with a lower CIR rate (HR, 0.37; 95% CI 0.13–1.08; p = 0.07). In contrast, correlations were detected between the incidence of both small and moderate-large ascites and a higher NRM rate (HR, 2.80; 95% CI 1.09–7.19; p = 0.03 for small ascites and HR, 4.31; 95% CI 1.75–10.63; p < 0.01 for moderate-large ascites). Additionally, we compared the OS rate between patients who responded to treatment for ascites and those who did not. In patients with and without clear causes of ascites in whom it was possible to assess treatment effects, the attenuation of ascites was confirmed in 27 out of 38. No significant difference was observed in the OS rate (p = 0.09) between these groups; however, it was slightly higher in patients showing the attenuation of ascites (Fig. 2).
Clinical outcomes by the status of ascites within 90 days of allo-HSCT. (A) Overall survival, (B) cumulative incidence of relapse, and (C) non-relapse mortality were stratified according to the status of ascites within 90 days of allo-HSCT (no vs. small vs. moderate-large).

Clinical outcomes according to responses to treatment for ascites within 90 days of allo-HSCT. Overall survival was stratified according to responses to treatment for ascites within 90 days of allo-HSCT (no vs. yes).
Correlations were also observed between a lower OS rate and being female as well as having a high/very high refined DRI, while a higher OS rate correlated with the use of ATG. A higher CIR rate correlated with a high/very high refined DRI, while a lower CIR rate correlated with MMRD/MMUD. The following causes of NRM (n = 42) were identified: infectious disease in 12 patients (29%), GVHD in 13 (31%), organ failure in 3 (7%), secondary malignancy in 2 (5%), bleeding in 3 (7%), TMA in 5 (12%), engraftment failure in 3 (7%), and suicide in 1 (2%).
Additionally, 41 out of 58 patients with ascites died during the follow-up period. The following causes of death were identified: disease recurrence in 12 patients (29%), infectious disease in 7 (17%), GVHD in 9 (22%), organ failure in 3 (7%), secondary malignancy in 1 (2%), bleeding in 4 (10%), TMA in 3 (7%), engraftment failure in 1 (2%), and suicide in 1 (2%).
Discussion
Limited information is currently available on the potential factors associated with ascites after allo-HSCT; therefore, a comprehensive analysis of potential factors was performed herein. We previously examined potential factors for and the prognostic impact of pericardial effusion after allo-HSCT16. Based on the findings obtained, we herein investigated potential factors for ascites after allo-HSCT. The multivariate analysis identified VOD/SOS and MAC as potential factors for small ascites within 90 days of allo-HSCT. G2-4 acute GVHD was also associated with the development of ascites. G2-4 acute GVHD, VOD/SOS, and MAC were regarded as acceptable potential factors for ascites after allo-HSCT. Previous studies reported that acute GVHD1 and VOD/SOS17,18 exhibited the clinical sign of ascites for a number of reasons, such as inflammation and sinusoidal endothelial cell injury. Although MAC is associated with intensive donor immune suppression and antitumor effects, cardiac and hepatic toxicities (including VOD/SOS) are caused by cyclophosphamide, total body irradiation, and busulfan included in MAC19,20,21,22. Organ injury by MAC may show the clinical sign of ascites. In the present study, TMA correlated with moderate-large ascites after allo-HSCT. Furthermore, a previous study reported that TMA induced pericardial and pleural effusion23,24. Nevertheless, limited information is currently available on the relationship between TMA and ascites25. TMA damages multiple organs, including the heart, kidneys, and gut23,24. Organ failure caused by TMA may manifest the clinical sign of ascites. The present results indicate that ascites, particularly moderate-large ascites, reflects the presence of TMA. TMA and VOD/SOS share a common pathogenesis of vascular endothelial syndromes that develop early after HSCT26. Vascular endothelial cell damage induces TMA and VOD/SOS, which cause renal, cardiac, and gut or liver damage, ultimately leading to multiple organ failure. Nevertheless, the clinical sign of ascites is not included in the diagnostic criteria of TMA11,12, whereas it is one of the diagnostic criteria of VOD/SOS4,5. Therefore, ascites may be an important clinical sign in not only VOD/SOS, but also TMA.
A recent study reported a patient who developed transudative refractory ascites secondary to non-cirrhotic, non-VOD/SOS-related portal hypertension after allo-HSCT27. Since the NRM rate of this disease entity is 63%, it was concluded to be a previously unrecognized and fatal complication after allo-HSCT. Further studies on the underlying causes of this disease entity are warranted.
Ascites has a negative impact on the NRM rate of allo-HSCT. We investigated the effects of ascites on transplant outcomes. The multivariate analysis revealed correlations between the incidence of both small and moderate-large ascites and poor OS and higher NRM rates. G2-4 acute GVHD was associated with the development of small ascites. Previous studies showed that the rate of CIR was lower in patients with G2-4 acute GVHD28. In the present study, small ascites induced by G2-4 acute GVHD was associated with a slightly lower CIR. On the other hand, TMA was the only potential factor for moderate-large ascites. Therefore, moderate-large ascites did not correlate with CIR.
There are a number of limitations that need to be addressed. This was a retrospective analysis that may have had a selection bias. Fifty out of the 260 patients recruited were excluded due to a lack of data on ascites and the small subset group. CT and abdominal ultrasound were conducted on patients with negative clinical signs. Since the times at which these clinical examinations were performed varied, the incidence of ascites may have been underestimated. Furthermore, we excluded patients who died within 90 days of allo-HSCT from the analysis. This study design may also have influenced analysis outcomes. Another limitation is the small number of ascites events, which may have affected the potential factor analysis.
In conclusion, the present results identified VOD/SOS and MAC as significant potential factors for small ascites within 90 days of allo-HSCT. TMA was associated with moderate-large ascites. Lower OS and higher NRM rates correlated with the incidence of both small and moderate-large ascites. The present study is novel because it investigated potential factors for ascites after allo-HSCT as well as the impact of the amount of ascites on the prognosis of patients. A relationship was observed between ascites, particularly moderate-large ascites, and TMA. Further investigation is warranted to establish whether the clinical sign of ascites improves the diagnostic quality of TMA in a large-scale study.
Data availability
The data supportive the findings of this study are available on request to the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
-
Seber, A., Khan, S. P. & Kersey, J. H. Unexplained effusions: Association with allogeneic bone marrow transplantation and acute or chronic graft-versus-host disease. Bone Marrow Transplant. 17, 207–211 (1996).
Google Scholar
-
Jagasia, M. H. et al. National institutes of health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. The 2014 diagnosis and staging working group report. Biol. Blood Marrow Transplant. 21, 389–401 (2015).
Google Scholar
-
McDonald, G. B. et al. Veno-occlusive disease of the liver and multiorgan failure after bone marrow transplantation: A cohort study of 355 patients. Ann. Intern. Med. 118, 255–267 (1993).
Google Scholar
-
Jones, R. J. et al. Venoocclusive disease of the liver following bone marrow transplantation. Transplantation 44, 778–783 (1987).
Google Scholar
-
Mohty, M. et al. Revised diagnosis and severity criteria for sinusoidal obstruction syndrome/veno-occlusive disease in adult patients: A new classification from the European Society for Blood and Marrow Transplantation. Bone Marrow Transplant. 51, 906–912 (2016).
Google Scholar
-
Bacigalupo, A. et al. Defining the intensity of conditioning regimens: Working definitions. Biol. Blood Marrow Transplant. 15, 1628–1633 (2009).
Google Scholar
-
Giralt, S. et al. Reduced-intensity conditioning regimen workshop: Defining the dose spectrum. Report of a workshop convened by the center for international blood and marrow transplant research. Biol. Blood Marrow Transplant. 15, 367–369 (2009).
Google Scholar
-
Armand, P. et al. Validation and refinement of the disease risk index for allogeneic stem cell transplantation. Blood 123, 3664–3671 (2014).
Google Scholar
-
Glucksberg, H. et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation 18, 295–304 (1974).
Google Scholar
-
Przepiorka, D. et al. 1994 consensus conference on acute GVHD grading. Bone Marrow Transplant. 15, 825–828 (1995).
Google Scholar
-
Ho, V. T. et al. Blood and marrow transplant clinical trials network toxicity committee consensus summary: Thrombotic microangiopathy after hematopoietic stem cell transplantation. Biol. Blood Marrow Transplant. 11, 571–575 (2005).
Google Scholar
-
Ruutu, T. et al. Diagnostic criteria for hematopoietic stem cell transplant-associated microangiopathy: Results of a consensus process by an International Working Group. Haematologica 92, 95–100 (2007).
Google Scholar
-
Anderson, J. R., Cain, K. C. & Gelber, R. D. Analysis of survival by tumor response. J. Clin. Oncol. 1, 710–719 (1983).
Google Scholar
-
Klein, J. P., Rizzo, J. D., Zhang, M. J. & Keiding, N. Statistical methods for the analysis and presentation of the results of bone marrow transplants. Part 2: Regression modeling. Bone Marrow Transplant. 28, 1001–1011 (2001).
Google Scholar
-
Kanda, Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant. 48, 452–458 (2013).
Google Scholar
-
Kubo, H. et al. Risk factors for and the prognostic impact of pericardial effusion after allogeneic hematopoietic stem cell transplantation. Transplant. Cell. Ther. 27(949), e1-949.e8 (2021).
-
Shulman, H. M., Fisher, L. B., Schoch, H. G., Henne, K. W. & McDonald, G. B. Veno-occlusive disease of the liver after marrow transplantation: Histological correlates of clinical signs and symptoms. Hepatology 19, 1171–1181 (1994).
Google Scholar
-
Vreuls, C. P. et al. Sinusoidal obstruction syndrome (SOS): A light and electron microscopy study in human liver. Micron 84, 17–22 (2016).
Google Scholar
-
Goldberg, M. A., Antin, J. H., Guinan, E. C. & Rappeport, J. M. Cyclophosphamide cardiotoxicity: An analysis of dosing as a risk factor. Blood 68, 1114–1118 (1986).
Google Scholar
-
Floyd, J. D. et al. Cardiotoxicity of cancer therapy. J. Clin. Oncol. 23, 7685–7696 (2005).
Google Scholar
-
McDonald, G. B. et al. Cyclophosphamide metabolism, liver toxicity, and mortality following hematopoietic stem cell transplantation. Blood 101, 2043–2048 (2003).
Google Scholar
-
Yakushijin, K. et al. Sinusoidal obstruction syndrome after allogeneic hematopoietic stem cell transplantation: Incidence, risk factors and outcomes. Bone Marrow Transplant. 51, 403–409 (2016).
Google Scholar
-
Khosla, J., Yeh, A. C., Spitzer, T. R. & Dey, B. R. Hematopoietic stem cell transplant-associated thrombotic microangiopathy: Current paradigm and novel therapies. Bone Marrow Transplant. 53, 129–137 (2018).
Google Scholar
-
Young, J. A., Pallas, C. R. & Knovich, M. A. Transplant-associated thrombotic microangiopathy: Theoretical considerations and a practical approach to an unrefined diagnosis. Bone Marrow Transplant. 56, 1805–1817 (2021).
Google Scholar
-
Jodele, S. et al. A new paradigm: Diagnosis and management of HSCT-associated thrombotic microangiopathy as multi-system endothelial injury. Blood Rev. 29, 191–204 (2015).
Google Scholar
-
Carreras, E. & Diaz-Ricart, M. The role of the endothelium in the short-term complications of hematopoietic SCT. Bone Marrow Transplant. 46, 1495–1502 (2011).
Google Scholar
-
Varma, A. et al. Idiopathic refractory ascites after allogeneic stem cell transplantation: A previously unrecognized entity. Blood Adv. 4, 1296–1306 (2020).
Google Scholar
-
Kanda, Y. et al. Effect of graft-versus-host disease on the outcome of bone marrow transplantation from an HLA-identical sibling donor using GVHD prophylaxis with cyclosporin A and methotrexate. Leukemia 18, 1013–1019 (2004).
Google Scholar
Acknowledgements
We thank the medical, nursing, data processing, laboratory, and clinical staff at our institutions (Faculty of Medicine, Kagawa University, Kagawa, Japan and Takamatsu Red Cross Hospital, Kagawa, Japan) for their contributions to the present study and their care towards patients.
Author information
Authors and Affiliations
Contributions
H.K., O.I., and T.F. contributed to the study concept and design. H.K. carried out the statistical analyses and wrote the manuscript. H.K., T.I., and Y.H.K. collected data. Y.K., J.K., M.U., H.F. and N.K. provided critical reviews and edits. All authors read and approved the manuscript as submitted.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
Reprints and Permissions
About this article
Cite this article
Kubo, H., Imataki, O., Fukumoto, T. et al. Potential factors for and the prognostic impact of ascites after allogeneic hematopoietic stem cell transplantation.
Sci Rep 13, 13005 (2023). https://doi.org/10.1038/s41598-023-39604-6
-
Received: 04 January 2023
-
Accepted: 27 July 2023
-
Published: 10 August 2023
-
DOI: https://doi.org/10.1038/s41598-023-39604-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.