Cancer and neoplasms
Updated perspectives on the diagnosis and management of FAP
Filippos Kyriakidis,1,* Dionysios Kogias,2,* Theodora Maria Venou,1,* Eleni Karlafti,3,4 Daniel Paramythiotis5
1Second Chemotherapy Department, Theagenio Cancer Hospital of Thessaloniki, Thessaloniki, Greece; 2First Department of Internal Medicine, University General Hospital of Alexandroupolis, Alexandroupolis, Greece; 3Emergency Department, AHEPA General University Hospital, Aristotle University of Thessaloniki, Thessaloniki, Greece; 4First Propaedeutic Department of Internal Medicine, University General Hospital of Thessaloniki AHEPA, Aristotle University of Thessaloniki, Thessaloniki, Greece; 5First Propaedeutic Surgery Department, AHEPA University General Hospital of Thessaloniki, Aristotle University of Thessaloniki, Thessaloniki, Greece
Correspondence: Filippos Kyriakidis, Tel +30 6984996573, Email [email protected]
Abstract: Familial adenomatous polyposis (FAP) is an autosomal dominant cancer predisposition syndrome marked by extensive colorectal polyposis and a high risk of colorectal cancer (CRC). Having access to screening and enrollment programs can improve survival for patients with FAP by enabling them to undergo surgery before the development of colorectal cancer. Provided that there are a variety of surgical options available to treat colorectal polyps in patients with adenomatous polyposis, the appropriate surgical option for each patient should be considered. The gold-standard treatment to reduce this risk is prophylactic colectomy, typically by the age of 40. However, colectomy is linked to morbidity and constitutes an ineffective way at preventing extra-colonic disease manifestations, such as desmoid disease, thyroid malignancy, duodenal polyposis, and cancer. Moreover, extensive studies have been conducted into the use of chemopreventive agents to prevent disease progression and delay the necessity for a colectomy as well as the onset of extracolonic disease. The ideal chemoprevention agent should demonstrate a biologically plausible mechanism of action and provide safety, easy tolerance over an extended period of time and a lasting and clinically meaningful effect. Although many pharmaceutical and non-pharmaceutical products have been tested through the years, there has not yet been a chemoprevention agent that meets these criteria. Thus, it is necessary to develop new FAP agents that target novel pathways, such as the mTOR pathway. The aim of this article is to review the prior literature on FAP in order to concentrate the current and future perspectives of diagnosis and treatment. In conclusion, we will provide an update on the diagnostic and therapeutic options, surgical or pharmaceutical, while focusing on the potential treatment strategies that could further reduce the risk of CRC.
Keywords: FAP, colorectal cancer, surveillance, genetic testing, surgery, chemoprevention
Introduction
Familial adenomatous polyposis (FAP), inherited in an autosomal dominant manner, is marked by the occurrence of hundreds to thousands of colorectal adenomatous polyps from early adolescence until the third decade of life, resulting in almost certain colorectal cancer development by the age of fifty years in the absence of prophylactic surgery.1–3 Based on the available data, the prevalence of FAP is 2.29–3.2 cases per 100,000 individuals.4 Moreover, FAP is responsible for almost 0.5% to 1% of all cases of colorectal cancer and affects both men and women equally.5,6 Adenomatous polyposis was first described histologically in 1881 by Sklifasowski.3 He described the case of a 51-year-old merchant who had been suffering from bloody diarrhea and abdominal pain for seven years. Adenomas were identified after histological examination of large polyps, removed during an operation.7 As a variant of FAP disease, attenuated FAP (aFAP) usually affects individuals over 40 years of age and has less than a hundred colonic adenomas. Milder aFAP phenotype is characterized by fewer late-onset adenomas that can be managed endoscopically, and as a result, surgery can be delayed for a significant amount of time.1
FAP is a hereditary syndrome with potential manifestations alongside the whole gastrointestinal system.8 The most commonly seen extracolonic malignancy in patients with FAP is duodenal cancer, which is the leading cause of death after prophylactic colectomy. The cumulative risk of duodenal cancer in patients with FAP is approximately 4.5% at 57 years old and rises to 18% at 75 years old8. Patients with FAP can also develop other extraintestinal complications, such as congenital hypertrophy of the retinal pigment epithelium (CHRPE) (60%), desmoid tumors (20%), thyroid papillary carcinomas (1–2%), medulloblastomas (1–2%), hepatoblastomas (1–2%), osteomas (20%), and benign skin lesion.9–11 In aFAP, the development of extraintestinal malignancies is less likely compared to classic FAP.5 There are even rare cases where FAP or aFAP coexist with uncommon disease entities, such as multiple GIST, highlighting the need for more research in this field.12
FAP and aFAP result from pathogenic (P) or likely pathogenic (LP) germline mutations of the APC (Adenomatous polyposis coli) gene on chromosome 5q2, contributing to approximately 1% of colorectal cancers.2,13 A dominant inheritance pattern is observed in classic FAP, and the penetration of the mutation reaches 100%.2,5,14,15 To date, over 1500 different mutations in the APC gene have been found till now.16 The APC mutation is detected in about 80% of patients with FAP who undergo protein-truncating tests,17 while genetic testing identifies it in 85% of patients.18
The APC protein is a 312-kDa tumor suppressor, which is implicated in many cellular processes, such as intercellular adhesion, signal cascades, multiplication, cell death, and migration, having a crucial role in Wnt signaling. Wnt proteins are associated with many developmental events during embryogenesis as well as in tissue homeostasis in adult organisms.3 One of the significant roles of APC gene is the regulation of intracellular levels of β-catenin, a protein responsible for cell adhesion and extracellular signal transduction, having a negative regulatory effect on Wnt signaling.19 In particular, expression of APC gene is associated with phosphorylation, ubiquitination, and subsequent proteolytic degradation of b-catenin.20,21 Furthermore, APC expression was associated with inhibited cell growth by inducing cell apoptosis in the study of Morin et al.22
Desmoid tumors are nonmetastatic, mostly sporadic, yet locally invasive tumors, which are similar to fibromatoses like Dupuytren’s contracture.1,23 However, 7.5–16% of FAP cases are hereditary, affecting 20% of patients.1,24 FAP-related types tend to manifest with larger, multifocal tumors and show greater recurrence and mortality rates.1 The majority of them seem to be triggered by surgical trauma, although they can occur “spontaneously”.24 They are most commonly found in the small intestine mesentery, while large fibrous masses develop in many cases.1
Carcinogenesis cascade resembles that of sporadic colorectal cancer (CRC). Several mechanisms can contribute to colorectal cancer, including chromosomal and microsatellite instability, as well as methylation of CpG islands. As a matter of fact, in the chromosomal instability pathway involving 85% of the sporadic tumors, carcinogenesis starts with mutations in the APC gene, followed by mutations in the KRAS (Kirsten Rat Sarcoma Viral) gene and subsequently in the TP53 (Tumor p53) gene.25 APC mutations are present in about 80% of FAP tumors, and 60% lack the full-length APC protein. Similarly, in FAP, the development of adenomas is caused by the damage to a second previously normal allele. Progression from adenoma to CRC occurs after APC gene inactivation, in the presence of mutations in other genes such as KRAS and TP53 at a later stage of carcinogenesis.2
Most of these germline mutations are found in a hot spot region of the APC gene between codons 1055 and 1309 in the 5` of exon 15. As distinct from the germline hotspot region, somatic mutations are observed in the mutation cluster region (MCR), which contains codons between 1250 and 1450.2 The number of colonic adenomas depends on where mutations occur in the APC gene5 (Figure 1). In patients with aFAP, APC gene mutations are located in 3 distinct locations: the far proximal (5′) end of the gene (5 first exons, codon 78 to 167), the far distal (3′) end of the gene (codon 1581 to 2843), or in locations of exon 9 (codon 312 to 412). There is evidence that mutations in the 3′ APC gene are associated with higher incidence of desmoid tumors2,5 (Figure 1). In patients with FAP, mutations located beyond codon 1309 and codon 1444 increased the risk of desmoid tumor development 17-fold and 12-fold, respectively, compared with mutations located in genetic loci close to the far proximal end of the APC gene.26
 |
Figure 1 Loci of mutations in the APC gene and genotype-phenotype associations. |
Nowadays, there are still many patients diagnosed with cancer on a background of FAP for three main reasons: 1. adoption and non-paternity (unknown family history), 2. non-compliance to screening and scheduled follow-up sessions in the context of a positive family history or after a prophylactic surgery (development of malignancy in the remaining rectum or in the ileoanal pouch or rectal cuff) and finally 3. de novo mutations (absence of family history).1
The aim of the present review is to present the new perspectives in the field of Familial Adenomatous Polyposis diagnosis and overall management (surgical management and chemoprevention).
Diagnosis of FAP
The suspicion of FAP diagnosis arises in the presence of primarily developed adenomas (no less than 100 in number) in the large intestine.27 The number of patients who get diagnosed with FAP between 10 and 20 years of life has been increased over time thanks to genetic testing and detection of the specific variant of the family, or screening family members at risk using endoscopy.28 Diagnosis of FAP or aFAP is based on the detection of a P/LP variant in the APC gene.27 More specifically, mutations in the APC gene, located between codons 1250 and 1464 (especially codon 1309) are associated with more severe diseases in contrast with mutations in the C-terminal domain.29
According to National Comprehensive Cancer Network (NCCN) guidelines, single-site testing is suggested in case of a known P/LP variant in the APC gene in the family. The use of multi-gene panels is preferred to test for a hereditary polyposis syndrome, when it is suspected, and a familial P/LP variant is not detected. A germline test should be used for the screening of extracolonic symptoms, counseling, risk assessment and family testing, as well as it is essential for the differential diagnosis between FAP and other disorders, such as MUTYH-related Polyposis (MAP), or POLD1 and POLE related polyposis (DNA polymerase genes).27 Moreover, a recent study supports that the effectiveness of clinical exome sequencing (CES) as regards the detection of P/LP variants or variants of unknown significance (VUS) is not significantly different when compared to multi-gene panel testing for the diagnosis of hereditary polyposis syndromes or colorectal cancer. Nevertheless, this study was not designed exclusively for the diagnosis of FAP.30
Also, somatic mosaicism has been encountered in patients with FAP and therefore genetic testing in biological samples other than blood, such as a polyp, is recommended in several cases.31
In case that the P/LP variant has already been detected in a family, counseling, and screening of the remaining at-risk individuals are suggested. Generally, genetic testing for FAP diagnosis in a population of at-risk underage family members should begin in the age of 10 to 15 years old, when endoscopic surveillance begins.27
The diagnosis of classic FAP is set in the presence of heterozygosity for one pathogenic variant in the APC gene, as detected with genetic testing, and one of the following criteria:
- Over than (or equal) 100 adenomatous polyps in the colon or rectum (younger patients or patients with colectomies may present with less than 100 colorectal adenomatous polyps),
- A large number, but less than 100 adenomatous polyps in the colon or rectum and a verified diagnosis of FAP in a family member.4
Similarly, the diagnosis of aFAP is set in the presence of heterozygosity for one pathogenic variant in the APC gene, as detected with genetic testing, and:
- A family member with verified aFAP, and/or
- Less than 100 adenomatous polyps in the colon/rectum or
- More than 100 adenomatous polyps in the colon/rectum for patients older than 40 years old.4
Even though conventional endoscopy is the method commonly used in clinical practice for the diagnosis of FAP and the follow-up of the patients, there are also other methods with promising results, as presented below.32 According to Mortensen et al, fluorescence endoscopy has been demonstrated to increase the diagnostic value of conventional endoscopy in terms of detecting neoplasia, adenomas, and evaluating tumor invasion, although more studies of higher quality are needed to produce more firm results.33 Worth mentioning is fluorescence molecular endoscopy, a combination of fluorescence endoscopy with the use of cancer-specific probes (for example, labeled monoclonal antibodies), which is a very promising alternative with great potential for the future.34
In a study where fluorescence endoscopy was compared to chromoendoscopy, a different endoscopic technique that uses dyes to highlight suspected neoplasms in the GI tracts’ mucosa, the first had a significantly higher diagnostic accuracy for depth of cancerous invasion (89% vs 68%).35 With chromoendoscopy using indigo carmine uniformly sprayed on the mucosa of the duodenum and a dedicated catheter, the mucosal abnormalities are highlighted. Numerous studies have shown the effectiveness of this inexpensive, easy-to-use method.36 For instance, there is evidence that indigo carmine chromoendoscopy improved the diagnosis of adenomas of the duodenum, allowing the Spigelman stage to be raised in 51 cases of FAP in a monocentric prospective study.37
Surveillance of FAP
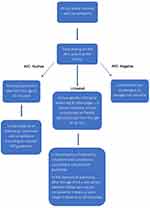
The role of active surveillance is important and can even constitute a viable alternative to immediate treatment in some cases.24 According to several guidelines, surveillance in FAP by sigmoidoscopy/colonoscopy should be performed every one to three years starting at the age of 10 to 14 years.38 Affected individuals without P/LP variants detected in both their test and their family are subject to the same surveillance as untested individuals (for instance, those who refuse for personal reasons to be tested) with known P/LP variant in their family. In the context of surveillance, children between the ages of 10 and 15 years should undergo high-quality colonoscopies (preferably) or flexible sigmoidoscopies every 12 months. Following 15 years of age, surveillance intervals should be increased to every 2 years if results remain negative. Depending on clinical judgment, the interval may be prolonged further in case of multiple surveillance exams without adenomas.27 The basic principles of genetic testing and surveillance of asymptomatic family members with a known pathogenic APC variant in their family are illustrated in the diagram (Figure 2).27
|
Figure 2 Genetic testing and surveillance of individuals with a known pathogenic APC variant in their family. |
Regarding duodenal polyposis there are four different stages, ranging from stage 0 to IV, determined by the Spigelman stage.38 The Spiegelman duodenal surveillance score was evaluated by Sourrouille et al in 2017, especially in relation to high-grade dysplasia. As indicated by a multivariate analysis, high-grade dysplasia was independently associated with age at the first endoscopy as well as alterations to the papilla (size and gross aspect).39
According to international guidelines from NCCN and European Society of Gastrointestinal Endoscopy (ESGE), it is recommended carry out forward and side view esophagogastroduodenoscopy to accurately assess the ampullary and periampullary regions beginning at age 20–25 years old (as upper gastrointestinal polyps typically develop 7–10 years after colon polyps),29 or earlier in case that colectomy is performed before the age of 20, then a follow-up endoscopy should be performed based on the Spigelman stage.8 Nonetheless, the concept of early screening for FAP is supported by some reports describing upper gastrointestinal (GI) tract involvement in FAP children.40,41 As the prevalence of duodenal adenomatosis increases with age, the vast majority of FAP patients will obtain duodenal adenomas during their lifetimes.42 Due to the evidence that duodenal involvement occurs earlier than expected in children, suitable surveillance strategies should be implemented, considering Spigelman staging, thus aiming to halt the progression of duodenal lesions to adenocarcinomas.43
Further surveillance of the small bowel may be advised in cases of advanced Spigelman stage. Nevertheless, performing capsule endoscopy (CE) or magnetic resonance enteroclysis in all FAP patients, including patients with non-clinically significant small bowel polyps, does not seem reasonable, until the importance of non-duodenal polyps is illustrated.8 According to the American College of Gastroenterology (ACG), it is not clear whether the use of Computed Tomography (CT) enterography, balloon enteroscopy, or CE examinations of the small intestine outside the range of the upper endoscope is indicated, when upper endoscopy reveals severe duodenal polyposis; however, the guidelines do not provide specific indications of surveillance.44
An abdominal MRI should be performed after colectomy every 3 to 5 years in patients with a family history of desmoid tumors. It is also crucial that patients with FAP undergo thyroid cancer surveillance, as they are more likely to develop papillary thyroid cancer (particularly cribriform-morular variant), which is more likely to occur in female patients, so thyroid tests or ultrasounds should begin at an early age. If more than 1 to 2 adenomatous polyps are present in childhood, regardless of family history, testing for underlying APC variants is suggested.29
In order to identify hepatoblastoma, which has a small correlation with FAP, abdominal ultrasound surveillance and serum a-fetoprotein measurements should be performed in children from the first month after birth and every three to six months until the age of 4 years old and have also been recommended in certain families with a history of hepatoblastoma.45
As polyps continue to accumulate in the retained rectum after ileorectal or ileosigmoid anastomosis (IRA/ISA), and in the pouch after ileal pouch-anal anastomosis (IPAA), patients remain at risk for cancer development after colectomy. Monitoring disease progression, removing lesions before they become malignant, and preventing surgical procedures that could otherwise be avoided remain the primary goal of lifelong endoscopic surveillance. As a standard of care, rectum or pouch polyposis should be monitored every half a year to three years, based on the severity of the polyposis, during a GI tract endoscopy of a patient with FAP. The most commonly observed gastric lesion is a fundic gland polyp, which occurs in 65 to 88% of cases.38 Although the possibility of cancer development is low in these lesions, Mankaney et al reported that the number of patients diagnosed with FAP and gastric malignancy has been increased.46 As of yet, there is no consensus on the best time to resect gastric adenomas in FAP or the preferred technique to use.38
Surgical Management of FAP
Managing families and individuals affected by FAP by endoscopic and imaging techniques aims to prevent cancer, while surgery’s primary purpose is to treat cancer and prevent death associated with colorectal cancer with no effect on life quality. Surgery in FAP will be as necessary as in any other cancer.1,47 Currently, there are no standardized guidelines regarding the type and exact operation time in patients with FAP.47
Colorectal adenomas can be safely monitored exclusively with endoscopy, a decision depending on factors like age, size, number, and histology.38 Such factors also influence the decision to perform prophylactic surgery. Multiple polyps with a size over 10mm, polyps with a high-grade dysplastic character, and rapid multiplication of polyps warrant colectomy. Polyps exceeding 5mm in size, villous components, or high-grade dysplasia, however, may not require surgery.47 Nevertheless, the decision whether to undergo a colectomy is also dependent on other socioeconomic factors or the risk of developing a desmoid tumor.38,47
The age limit for preventive surgery is generally lowered from three decades in English-speaking countries to twenty years in Europe because of the elevated cancer risk after that age.47 As a result, in a recent study, researchers propose preventive surgery before 27 years of age for people with classic FAP to minimize the possibility of colorectal cancer. The risk increases from 10% to 25% between 28 and 32 years of age. On the contrary, prophylactic surgery before the age of 31 years old is recommended in cases of aFAP, where cancer development peaks later.48
The surgical options are 1. subtotal or total colectomy with ileorectal or ileosigmoid anastomosis, 2. restorative proctocolectomy (ileal pouch-anal anastomosis), and 3. proctocolectomy with end ileostomy.27,38,47 Removal of the rectum is not obligatory in the case of colorectal cancer. Overall, there is a 6.1–11.2% chance of rectal cancer following an ileorectal anastomosis in FAP. It is estimated that 1.1–1.9% of patients who undergo proctocolectomy will develop cancer, with the majority of malignancies occurring in the rectal cuff.38 In Table 1, indications, as well as advantages and disadvantages of each surgical procedure are presented based on the most recent NCCN guidelines (2022).27
|
Table 1 Comparison of Three Different Operations, Used in FAP Management (Indications, Contraindications, Advantages, Disadvantages) According to the Most Recent NCCN Guidelines (2022)27 |
When surgery is decided in case that colorectal cancer is absent and the number of polyps is less than 1000 in the colon and less than 5 in the rectum, total colectomy with ileorectal anastomosis is recommended in both the classic and attenuated forms of FAP. Treatment is individualized, and the type of operation is under discussion in individuals with a number of polyps between 5 and 19 in the rectum.47
According to the American Society of Colorectal Surgeons, restorative proctocolectomy (with ileo-anal anastomosis) is indicated in the presence of 1. mutation in the “MCR” between codons 1250 and 1450 of the APC gene, 2. the presence of a number greater than 500 colonic adenomas, or 3. over 20 rectal adenomas and finally 4. rectal adenomas not approachable with endoscopy. For example, in the case of a patient with less than 20 rectal adenomas, which can all be endoscopically extracted and have mutations outside the “mutation cluster region”, colectomy with ileorectal anastomosis is the optimal surgical procedure.1
Other long-term outcomes should be considered in addition to cancer risk and reoperation after colectomy when choosing the appropriate surgery type. Among the crucial aspects are the effects on urological and bowel function, fertility, sexual function, and the overall quality of life. As for several complications, sexual dysfunction, and dietary restriction, Aziz et al concluded that there was no significant difference between ileorectal and ileal pouch-anal anastomosis in patients with FAP. Furthermore, they reported that rectal cancer had only developed in the group of patients with ileorectal anastomosis, but this group of patients seemed to have better fecal continence.49
Despite the association between total colectomy with ileorectal anastomosis and development of rectal cancer, there is less possibility of desmoid tumor development in this type of operation.47 However, based on the results of a recent meta-analysis, there is no statistically significant difference between patients with ileorectal anastomosis and a group of patients with ileal pouch-anal anastomosis as regards desmoid tumor development.50 On the other hand, in two multivariate analyses, it was found that ileal pouch-anal anastomosis increases the risk of development of desmoids (when other confounders are also taken into account).51,52
Finally, proctocolectomy with end ileostomy is preferred in several cases, such as a postpartum woman with problems of incontinence or in cases where there is a high risk of dysfunctional anastomosis.1 A final ileostomy is preferred in some rare cases of locally advanced cancer of the rectum, the presence of a large desmoid in the mesentery, or defective anal sphincter function.38
Total colectomy with ileorectal anastomosis is the first-line operation in most patients with aFAP without any signs of rectal involvement or polyposis which can be managed with endoscopy. In several patients with aFAP in which a large number of proximal polyps are present, the degree of resection is defined by the number and the location of the present adenomas. It is not recommended for patients with an excessive number of rectal polyps to undergo this type of operation.27
Chemoprevention of FAP
Prophylactic colectomy and endoscopic surveillance currently constitute the best choice for cancer prevention in FAP. However, drugs that can prevent CRC has received a lot of attention from researchers in recent years. Chemopreventive medicines work to reduce or delay the risk of cancer by targeting the pathways that lead to cancer development. Hereditary disorders are an appropriate environment for chemoprevention because chemopreventive effects may have a bigger impact on high-risk diseases. Several clinically significant endpoints for FAP chemoprevention have been identified over the years, including the number of polypectomies, the number of patients undergoing surgery, the total number of polyps (larger than 10 mm) removed, duodenal cancer, a decrease in the frequency of surveillance colonoscopies, and the number of patients who required polypectomies for polyps larger than 10 mm.53
Aspirin
According to recent ACG clinical guidelines on CRC screening, aspirin is suggested as a chemopreventive drug in the average risk population between 50 and 69 years old.5 CAPP1 trial (Colorectal Adenoma/Carcinoma Prevention Programme 1) constitutes the largest clinical trial for the use of aspirin, in which 227 patients with familial adenomatous polyposis were randomized to aspirin, aspirin plus resistant starch, resistant starch alone and placebo. The 133 patients who included in the primary analysis had no benefit of 600 mg of aspirin, 30 g of fermentable fiber, or both, as compared with placebo, regarding polyp burden. The only difference (p = 0.02) was the size reduction of the largest polyp among patients taking aspirin for more than 1 year.54 However, the early completion of the study constitutes a major limitation about its results.55 J-FAPP IV, a new randomized controlled clinical trial of 104 FAP patients with intact colon treated with aspirin/mesalazine, aspirin/mesalazine placebo, mesalazine/aspirin placebo, and placebo/placebo low-dose aspirin, revealed a significant reduction in the recurrence of polyps greater than 5 mm.56 Nevertheless, there is no well-documented recommendation for aspirin as a chemopreventive agent for FAP.
Celecoxib and Rofecoxib
It is well established that cyclooxygenase (COX), and more specifically COX-2, is essential for the development of gastrointestinal polyps.57 In colonic adenomas, COX-2 expression is increased, which is linked to adenoma characteristics indicative of malignant transformation.57 Celecoxib as well as rofecoxib are two of the most studied drugs among COX-2 inhibitors in FAP. In patients with polyposis syndromes, the COX-2 enzyme is upregulated in colonic cells and has a significant connection with APC and Wnt/β-catenin signaling: In APC knockout mice, this pathway was blocked to reduce the number of polyps.58,59 The use of celecoxib was first investigated for use in patients with FAP by Steinbach et al.60 Later, several clinical trials tested its efficacy, such as one double-blind, placebo-controlled study of 77 patients who were given celecoxib (100 or 400 mg twice daily) or placebo for six months resulting in a significant reduction in the number of polyps in a tattooed area of the colon.61 Also, a Phase I, dose-escalation trial in 2010 studied 18 pediatric patients that showed a significant reduction in the number of polyps when treated with celecoxib at a dose of 16 mg/kg/day.62 Of note, the endpoints of polyp burden and polyp number do not indicate changes in rates of colorectal cancer, colectomy, or death. A recent study concerning celecoxib’s efficacy of Yang et al showed that celecoxib’s bioavailability varies between normal and polyp tissues and that it may have an impact on how FAP patients respond clinically.63 However, other studies showed that selective COX-2 inhibitors have significant toxicities when used for a long time. A long-term, multicenter, randomized, placebo-controlled, double-blind trial of rofecoxib (25 mg daily) in comparison with placebo for prevention of colorectal cancer indicated a higher rate of thrombotic events after 18 months of treatment with COX-2 inhibitor in a group of patients aged above 40 years and having ≥1 colorectal adenoma removed in the 3 months prior to treatment initiation.64 Study data are compatible with an early increase in risk that persists for one year after stopping treatment. Another multicenter, randomised, placebo-controlled, double-blind trial (APPROVe study) proved that rofecoxib is associated with increased risk of cardiovascular and other adverse events.65 Based on these findings, Merck voluntarily withdrew rofecoxib (Vioxx) off the American market in 2004. Along with rofecoxib, celecoxib has been blamed for similar adverse events in the past. A meta-analysis of 6 placebo-controlled trials comparing celecoxib (3 dose regimens: 400 mg QD, 200 mg BID, or 400 mg BID) with placebo for conditions other than arthritis with a planned follow-up of at least 3 years revealed differential cardiovascular risk as a function of celecoxib dose regimen and baseline cardiovascular risk.66 There are no recent safety trials to study celecoxib in FAP as most of new research is focusing on other agents’ efficacy and safety.67 A recent meta-analysis of randomized controlled trials of Ye SY et al68 regarding the efficacy and safety of celecoxib combined with standard cancer therapy indicated that adding celecoxib to palliative therapy had a good safety profile. This leaves space for more research in the field of safety in case of celecoxib in the future. Finally, despite celecoxib ultimately being approved by the Food and Drug Administration for the treatment of colorectal polyps in patients with FAP, for now there are notable limitations that precluded widespread adoption of celecoxib as a long-term prophylactic agent.
Sulindac
Sulindac, a less-selective cyclooxygenase inhibitor with non-COX pathway effects, has been used for the clinical management of colorectal polyposis in FAP patients since 1993, when Giardiello et al69 performed the first randomized trial using sulindac alone. Although there was a reduction in polyp number and size, later studies failed to show a statistically significant benefit.70
Combination Therapies Using Cyclooxygenase Inhibitors
Difluoromethylornithine (DFMO) and erlotinib in combination with NSAIDs, particularly celecoxib and sulindac, constitute a regimen that has recently been tested for efficacy in patients with FAP.67 It inhibits polyamine metabolism, specifically ornithine decarboxylase – ODC. Erlotinib is an anti-EGFR drug approved for lung cancer, and several studies had demonstrated that the EGFR signaling in colonic crypts of APCMin ± mice had been upregulated.71 Also, erlotinib has been studied in association with sulindac in clinical trials regarding FAP syndrome.
Combined treatment with sulindac plus erlotinib was firstly tried by Baker et al72 in 2016 where it was shown that duodenal polyposis could be reduced by blocking EGFR and COX-2. Two years later, Delker et al73 revealed molecular evidence that the drug combination sulindac/erlotinib reached the intended tissue and was active in the expected pathways. Furthermore, drug-induced activation of innate immune pathways may have contributed to polyp regression. Additionally, in FAPEST trial which randomized 91 FAP patients to sulindac plus low-dose erlotinib versus placebo to assess differences in duodenal polyp burden at 6 months, sulindac plus erlotinib scheme showed a reduction in duodenal burden as well as a reduction in colorectal and pouch burden.74,75 However, several adverse events were reported limiting the use of this medical regimen at the dosing schedule applied in this study. Given that erlotinib’s pharmacokinetics may result in a lower side effect profile when dosed once per week, in 2022 it was conducted a Phase II trial of weekly erlotinib dosing in order to evaluate the Adverse Event (AE) profile, while still providing efficacy with respect to reduced polyp burden, in participants with FAP.76 After 6 months of management, the data demonstrated a decreased duodenal polyp burden and a moderately reduced lower GI polyp burden, with AEs of lower grade and well tolerated in 75% of the participants.
Although DFMO was firstly tested in association with sulindac in 2008 in a study that 375 patients with sporadic colorectal polyps showed a reduction in the recurrence of adenomas,77 this regimen was tested for the first time in FAP in 2020 in a clinical trial that there was not found any significant difference in preventing disease progression among different combinations of the drugs.78 In particular, Meyskens et al52 in 2008 proved that a low dose of DFMO plus sulindac at a dose one half the usual therapeutic dose significantly reduced the recurrence of all adenomas (70% decrease), advanced adenomas (92% decrease), and recurrence of more than one adenoma (95% decrease) in patients at moderately high risk for sporadic adenomas, while Burke et al53 in 2020 did not prove treatment benefit with combination therapy in the subgroup of patients who had duodenal polyposis, as well as there was no observed significantly lower incidence of FAP progression with the combination of eflornithine and sulindac than with either drug alone. Finally, a post-hoc analysis of a randomized clinical trial of the same year, where patients received either eflornithine (750 mg), sulindac (150 mg), or both once daily for up to 48 months, showed that the combination therapy compared to each drug alone offered an 80% risk reduction for disease progression.54 Also, patients who underwent major polypectomies (>10 mm) and had been administrated the combination therapy (750 mg eflornithine plus 150 mg sulindac) had a 100% risk reduction for disease progression, as none of these patients required a major surgery during a 48-month time period.79
When the combination between celecoxib and DFMO was tested in a randomised clinical chemoprevention trial in adults with FAP by Lynch et al, a significant reduction of colonic polyp burden at 6 months was found, while there was not noticed any significant difference in polyp count.80 Celecoxib plus DFMO also proved to have a manageable toxicity profile, thus setting a promising chemopreventive treatment, which, however, needs further clinical research.55
Fish Oil
Fish oil contains an omega-3 polyunsaturated fatty acid called Eicosapentaenoic acid (EPA), which has proved to have a remarkable antitumorigenic activity in vitro. In particular, EPA competitively binds to COX-2 enzyme producing pro-apoptotic and anti-inflammatory prostaglandins and reducing arachidonic acid metabolism, which is supposed to have a pro-tumorigenic and pro-inflammatory activity.81 When fish oil tested for 6 months in patients with FAP and ileorectal anastomosis, it significantly reduced the number of polyps and polyp burden between the treatment and the placebo group within a specific target area of the rectum.82 In our days, a randomized, double-blind, placebo-controlled study is planned to determine the efficacy in reduction of polypectomies and clinical disease progression, safety, and tolerability of EPA in FAP patients with ileorectal anastomosis for a longer period (24 months) (NCT03806426).
Turmeric
Curcumin, a polyphenolic compound derived from the spice called turmeric, is well known in traditional Asian medicine for its antioxidant, anti-inflammatory and anti-apoptotic properties. It inhibits the polyamine metabolism and slows cancer development and progression.83 In a pilot research, curcumin and quercetin were used to treat 5 FAP patients who had ileorectal anastomosis and an ileal pouch. Quercetin was utilized to improve curcumin absorption because of its poor absorption in the gut. All patients’ polyp size and number significantly decreased following the treatment time.84 A recent clinical trial who studied the curcumin consumption as a therapy by 44 FAP patients with either intact colon, ileorectal anastomosis and ileal pouch in comparison with placebo did not found significant differences in mean polyp number and size between the two groups.85
Ascorbic Acid
Ascorbic acid, or Vitamin C, has been associated with antineoplastic properties due to its antioxidant properties.86 Nonetheless, no studies have shown that ascorbic acid supplementation has a therapeutic advantage in reducing colorectal cancer in FAP patients. Several studies like the ones of Bussey et al87 and DeCosse et al88 failed to find statistically significant differences in number of polyps between groups when ascorbic acid compared alone with placebo. Key role in ascorbic acid failure played the fact that the toxicity to colorectal cancer cells is partially mediated through a KRAS or BRAF mutation.89 Yun et al90 found that therapy with ascorbic acid only decreased the frequency and size of intestinal polyps in mice with the KRAS mutation. As a result, ascorbic acid may be useful in individuals with FAP only after the KRAS mutation has been acquired. Ascorbic acid has limited usefulness as a chemoprevention medication in patients with FAP until this effect in individuals with KRAS mutations is proven in a clinical trial.
Rapamycin
Rapamycin, or sirolimus, is an immunosuppressive drug extensively used in transplant medicine whose function focuses on inhibiting mTOR pathway (mammalian target of rapamycin), which normally regulates cell division and cell proliferation enhancing cancer development and progression.38,91 Rapamycin is considered as a remarkable choice due to its immunomodulating activity even at a low dosage. In a pediatric study, two patients with FAP were administrated rapamycin obtaining reduction in size and grade of dysplasia of duodenal and colonic polyps. No adverse events were observed.92 Recently, a pilot study included 4 FAP patients with ileorectal anastomosis and ileal pouch with satisfactory results in reduction of the polyp number. However, there were several adverse events that downgraded its results.93
Mesalazine
First suggestion that a collision of phenotypes may influence the mutual presentation of inflammatory bowel diseases (IBD), especially Ulcerative Colitis (UC) and FAP, referred in 2012. Both of these conditions independently increase the risk of colorectal cancer.94 Mesalazine is the gold standard drug for treatment of UC. Recently, a study published by Ishikawa et al described 4 cases of FAP patients with UC that showed reduction of intestinal polyp diameter by mesalazine treatment, thus increasing its potential to suppress intestinal polyp development in FAP.95 Further studies need to be carried out in order to derive a reliable result. Last but not least, as mentioned above, mesalazine was also used as a treatment in J-FAPP IV study, which compared drug combinations of mesalazine with aspirin and placebo in FAP patients with intact colon and demonstrated a substantial reduction in the frequency of polyps larger than 5 mm in size.56
Metformin
Metformin has been used frequently for more than 50 years as an oral diabetic drug, is considered to be generally safe, and has recently gained interest due to its antineoplastic effects. Previous meta-analyses showed that people with type II diabetes who took metformin had a decreased risk of colorectal cancer.96,97 Given that metformin activates AMPK, which inhibits the mTOR pathway,98 a clinical trial was designed where a 7-month metformin treatment was given to patients with FAP syndrome. In comparison to placebo that regimen had no effect on the quantity or size of polyps in the colorectum or duodenum of FAP patients. These findings do not support the use of metformin to induce intestinal adenoma regression in FAP patients. However, colon polyps removed from the metformin-treated individuals had considerably lower mTOR signal (p-S6) expression than those removed from placebo-treated patients, which indicates a potential in this therapeutic model.99
Apart from the mTOR pathway which needs more research to yield a successful result, there are other ongoing trials studying treatment targets in FAP patients. In 2022, TUPELO trial examines the efficacy and safety of REC-4881, a MAP kinase inhibitor (NCT05552755) and another one is testing obeticholic acid (OCA), a drug similar to a bile acid the body makes (NCT05223036). Although in vitro and pre-clinical studies have shown that chemoprevention has a prospective and persuasive role in the treatment of FAP, according to the existing scientific literature reliable clinical data are still missing. As a result, FAP patients cannot receive any chemoprevention advice. To gather more clinical evidence, larger, well-predicted studies must be done in the future.
Conclusion
FAP is responsible for almost 1% of all CRC cases. Α patient with FAP has a very high probability of developing CRC but also may develop other extra colonic manifestations. This demonstrates the importance of early and accurate diagnosis and active surveillance, thus making the further improvement of diagnostic tools a necessity. Studies specifically designed to compare multi-gene panels and CES in the diagnosis of FAP are needed and the effort to identify more P/LP variants that remain currently unknown should continue. On the field of endoscopy, new methods such as fluorescence endoscopy, chromoendoscopy and even fluorescence molecular endoscopy have shown promising results, but more studies comparing the endoscopic techniques along with cost-benefit studies are needed to determine the role of each one in the diagnostic process. Furthermore, more research determining the significance of extracolonic manifestations of FAP and its associations with other disease entities may produce data that will impact the surveillance and treatment strategies in the future.
Many factors determine the choice of a specific surgical procedure, including the size of the polyps, the number of polyps in the colon or rectum, and the histology. The type and exact time of the operation in patients with FAP are not standardized at this time. Genetics and other socioeconomic factors should be considered when determining surgical management for each patient.
Regarding chemoprevention, any drug tested in a clinical trial to date, including aspirin, celecoxib, sulindac, combination sulindac/DFMO, combination celecoxib/DMFO, combination sulindac/erlotinib, fish oil, turmeric, and ascorbic acid, has failed to meet the ideals for an appropriate chemopreventive agent in FAP. Although mesalazine has shown encouraging results in reducing polyps in UC, more research is needed to prove its effectiveness in FAP. Targeting novel pathways, such as mTOR pathway, using rapamycin or metformin, has been shown to reduce the grade of dysplasia despite poor results and adverse events in clinical practice. The mTOR pathway along with two new treatment targets, REC-4881 and obeticholic acid, which are being investigated by an ongoing clinical study, are promising targets for the future. However, more clinical studies are needed to prove their efficacy in FAP.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Clark SK. Management of genetically determined colorectal cancer. Surgeon. 2019;17(3):165–171. doi:10.1016/j.surge.2019.03.003
2. Aghabozorgi AS, Ebrahimi R, Bahiraee A, et al. The genetic factors associated with Wnt signaling pathway in colorectal cancer. Life Sci. 2020:256. doi:10.1016/j.lfs.2020.118006
3. Short E, Sampson J. The role of inherited genetic variants in colorectal polyposis syndromes. Adv Genet. 2019;103:183–217. doi:10.1016/bs.adgen.2018.11.002
4. Yen T, Stanich P, Axell L. APC-Associated Polyposis Conditions; 1998.
5. Shaukat A, Kahi CJ, Burke CA, Rabeneck L, Sauer BG, Rex DK. ACG clinical guidelines: colorectal cancer screening 2021. Am J Gastroenterol Suppl. 2021;116(3):458–479. doi:10.14309/ajg.0000000000001122
6. Sokic-Milutinovic A. Appropriate management of attenuated familial adenomatous polyposis: report of a case and review of the literature. Digest Dis. 2019;37(5):400–405. doi:10.1159/000497207
7. Bülow S, Berk T, Neale K. The history of familial adenomatous polyposis. Fam Cancer. 2006;5(3):213–220. doi:10.1007/s10689-005-5854-0
8. Sanchez-Mete L, Stigliano V. Update on small bowel surveillance in hereditary colorectal cancer syndromes. Tumori. 2019;105(1):12–21. doi:10.1177/0300891618792461
9. Groen EJ, Roos A, Muntinghe FL, et al. Extra-intestinal manifestations of familial adenomatous polyposis. Ann Surg Oncol. 2008;15(9):2439–2450. doi:10.1245/s10434-008-9981-3
10. Burger B, Cattani N, Trueb S, et al. Prevalence of skin lesions in familial adenomatous polyposis: a marker for presymptomatic diagnosis? Oncologist. 2011;16(12):1698–1705. doi:10.1634/theoncologist.2011-0244
11. Short E, Thomas LE, Davies A, et al. APC transcription studies and molecular diagnosis of familial adenomatous polyposis. Eur J Hum Genet. 2020;28(1):118–121. doi:10.1038/s41431-019-0486-2
12. Paramythiotis D, Kyriakidis F, Karlafti E, et al. A rare case of multiple gastrointestinal stromal tumors coexisting with a rectal adenocarcinoma in a patient with attenuated familial adenomatous polyposis syndrome and a mini review of the literature. Medicina. 2022;58(8). doi:10.3390/medicina58081116
13. Galiatsatos P, Foulkes WD. Familial adenomatous polyposis. Am J Gastroenterol Suppl. 2006;101(2):385–398. doi:10.1111/j.1572-0241.2006.00375.x
14. Jansen AML, Goel A. Mosaicism in patients with colorectal cancer or polyposis syndromes: a systematic review. Clin Gastroenterol Hepatol. 2020;18(9):1949–1960. doi:10.1016/j.cgh.2020.02.049
15. Aretz S, Uhlhaas S, Caspari R, et al. Frequency and parental origin of de novo APC mutations in familial adenomatous polyposis. Eur J Hum Genet. 2004;12(1):52–58. doi:10.1038/sj.ejhg.5201088
16. Kadiyska TK, Todorov TP, Bichev SN, et al. APC promoter 1B deletion in familial polyposis-implications for mutation-negative families. Clin Genet. 2014;85(5):452–457. doi:10.1111/cge.12210
17. Ballhausen WG. Genetic testing for familial adenomatous polyposis. Ann N Y Acad Sci. 2000;910(1):36–49. doi:10.1111/j.1749-6632.2000.tb06699.x
18. Patel R, Hyer W. Practical management of polyposis syndromes. Frontline Gastroenterol. 2019;10(4):379–387. doi:10.1136/flgastro-2018-101053
19. Damalas A, Ben-Ze’ev A, Simcha I, et al. Excess β-catenin promotes accumulation of transcriptionally active p53. EMBO J. 1999;18(11):3054–3063. doi:10.1093/emboj/18.11.3054
20. Munemitsu S, Albert I, Souza B, Rubinfeld B, Polakis P, Bourne HR. Regulation of Intracellular (8-Catenin Levels by the Adenomatous Polyposis Coli (APC) tumor-suppressor protein communicated by. Proc Natl Acad Sci. 1995;92(7):3046–3050. doi:10.1073/pnas.92.7.3046
21. Orford K, Crockett C, Jensen JP, Weissman AM, Byers SW. Serine phosphorylation-regulated ubiquitination and degradation of β- catenin. J Biol Chem. 1997;272(40):24735–24738. doi:10.1074/jbc.272.40.24735
22. Morin PJ, Vogelstein B, Kinzlertt KW. Apoptosis and APC in Colorectal Tumorigenesis. Proc Natl Acad Sci. 1996;93(15):7950–7954. doi:10.1073/pnas.93.15.7950
23. Zhou MY, Bui NQ, Charville GW, Ghanouni P, Ganjoo KN. Current management and recent progress in desmoid tumors. Cancer Treat Res Commun. 2022;31. doi:10.1016/j.ctarc.2022.100562
24. Sanchez-Mete L, Ferraresi V, Caterino M, et al. Desmoid tumors characteristics, clinical management, active surveillance, and description of our fap case series. J Clin Med. 2020;9(12):1–14. doi:10.3390/jcm9124012
25. Tariq K, Ghias K, Tariq K, Ghias K. Colorectal cancer carcinogenesis: a review of mechanisms. Cancer Biol Med. 2016;13(1):120–135. doi:10.28092/j.issn.2095-3941.2015.0103
26. Bertario L, Russo A, Sala P, et al. Genotype and Phenotype Factors as Determinants of Desmoid Tumors in Patients with Familial Adenomatous Polyposis. Int J Cancer. 2001;95(2):102–107. doi:10.1002/1097-0215(20010320)95:23.0.CO;2-8
27. Gupta S, Weiss JM, Burke CA, et al. NCCN guidelines version 2.2022 Genetic/familial high-risk assessment: colorectal continue; 2022. Available from: https://www.nccn.org/home/. Accessed August 01, 2023.
28. Kennedy RD, Potter DD, Moir CR, El-Youssef M. The natural history of familial adenomatous polyposis syndrome: a 24 year review of a single center experience in screening, diagnosis, and outcomes. J Pediatr Surg. 2014;49(1):82–86. doi:10.1016/j.jpedsurg.2013.09.033
29. MacFarland SP, Zelley K, Katona BW, Wilkins BJ, Brodeur GM, Mamula P. Gastrointestinal Polyposis in Pediatric Patients. J Pediatr Gastroenterol Nutr. 2019;69(3):273–280. doi:10.1097/MPG.0000000000002421
30. Niu X, Amendola LM, Hart R, et al. Clinical exome sequencing vs. usual care for hereditary colorectal cancer diagnosis: a pilot comparative effectiveness study. Contemp Clin Trials. 2019:84. doi:10.1016/j.cct.2019.105820
31. Jelsig AM, Byrjalsen A, Madsen MB, et al. Novel genetic causes of gastrointestinal polyposis syndromes. Appl Clin Genet. 2021;14:455–466. doi:10.2147/TACG.S295157
32. Stanich PP, Sullivan B, Kim AC, Kalady MF. Endoscopic management and surgical considerations for familial adenomatous polyposis. Gastrointest Endosc Clin N Am. 2022;32(1):113–130. doi:10.1016/j.giec.2021.08.007
33. Mortensen OE, Nerup N, Thorsteinsson M, Svendsen MBS, Shiwaku H, Achiam MP. Fluorescence guided intraluminal endoscopy in the gastrointestinal tract: a systematic review. World J Gastrointest Endosc. 2020;12(10):388–400. doi:10.4253/wjge.v12.i10.388
34. Hartmans E, Tjalma JJJ, Linssen MD, et al. Potential red-flag identification of colorectal adenomas with wide-field fluorescence molecular endoscopy. Theranostics. 2018;8(6):1458–1467. doi:10.7150/thno.22033
35. Iseki K, Tatsuta M, Iishi H, Sakai N, Yano H, Ishiguro S. Effectiveness of the near-infrared electronic endoscope for diagnosis of the depth of involvement of gastric cancers. Gastrointest Endosc. 2000;52(6):755–762. doi:10.1067/mge.2000.110455
36. Amoyel M, Belle A, Dhooge M, et al. Endoscopic management of non-ampullary duodenal adenomas. Endosc Int Open. 2022;10(01):E96–E108. doi:10.1055/a-1723-2847
37. Hurley JJ, Thomas LE, Walton SJ, et al. The impact of chromoendoscopy for surveillance of the duodenum in patients with MUTYH-associated polyposis and familial adenomatous polyposis. Gastrointest Endosc. 2018;88(4):665–673. doi:10.1016/j.gie.2018.04.2347
38. Aelvoet AS, Buttitta F, Ricciardiello L, Dekker E. Management of familial adenomatous polyposis and MUTYH-associated polyposis; new insights. Best Pract Res Clin Gastroenterol. 2022;58–59. doi:10.1016/j.bpg.2022.101793
39. Sourrouille I, Lefèvre JH, Shields C, et al. Surveillance of duodenal polyposis in familial adenomatous polyposis: should the spigelman score be modified? Dis Colon Rectum. 2017;60(11):1137–1146. doi:10.1097/DCR.0000000000000903
40. Cohen S, Gorodnichenco A, Weiss B, et al. Polyposis syndromes in children and adolescents: a case series data analysis. J Nurs Adm. 2014;44(7–8):972–977. doi:10.1097/MEG.0000000000000143
41. Alkhouri N, Franciosi JP, Mamula P. Familial adenomatous polyposis in children and adolescents. J Pediatr Gastroenterol Nutr. 2010;51(6):727–732. doi:10.1097/MPG.0b013e3181e1a224
42. Bülow S, Björk J, Christensen IJ, et al. Duodenal adenomatosis in familial adenomatous polyposis. Gut. 2004;53(3):381–386. doi:10.1136/gut.2003.027771
43. Gutierrez Sanchez LH, Alsawas M, Stephens M, Murad MH, Absah I. Upper GI involvement in children with familial adenomatous polyposis syndrome: single-center experience and meta-analysis of the literature. Gastrointest Endosc. 2018;87(3):648–656.e3. doi:10.1016/j.gie.2017.10.043
44. Syngal S, Brand RE, Church JM, Giardiello FM, Hampel HL, Burt RW. ACG clinical guideline: genetic testing and management of hereditary gastrointestinal cancer syndromes. Am J Gastroenterol Suppl. 2015;110(2):223–262. doi:10.1038/ajg.2014.435
45. Katabathina VS, Menias CO, Khanna L, et al. Hereditary gastrointestinal cancer syndromes: role of imaging in screening, diagnosis, and management. Radiographics. 2019;39(5):1280–1301. doi:10.1148/rg.2019180185
46. Mankaney G, Leone P, Cruise M, et al. Gastric cancer in FAP: a concerning rise in incidence. Fam Cancer. 2017;16(3):371–376. doi:10.1007/s10689-017-9971-3
47. Menahem B, Alves A, Regimbeau JM, Sabbagh C. Colorectal family polyadenomatous diseases. What management in 2020? J Visc Surg. 2020;157(2):127–135. doi:10.1016/j.jviscsurg.2019.12.003
48. Kobayashi H, Ishida H, Ueno H, et al. Association between the age and the development of colorectal cancer in patients with familial adenomatous polyposis: a multi-institutional study. Surg Today. 2017;47(4):470–475. doi:10.1007/s00595-016-1398-1
49. Aziz O, Athanasiou T, Fazio VW, et al. Meta-analysis of observational studies of ileorectal versus ileal pouch-anal anastomosis for familial adenomatous polyposis. Br J Surg. 2006;93(4):407–417. doi:10.1002/bjs.5276
50. Xie M, Chen Y, Wei W, et al. Does ileoanal pouch surgery increase the risk of desmoid in patients with familial adenomatous polyposis? Int J Colorectal Dis. 2020;35(8):1599–1605. doi:10.1007/s00384-020-03578-y
51. Saito Y, Hinoi T, Ueno H, et al. Risk factors for the development of desmoid tumor after colectomy in patients with familial adenomatous polyposis: multicenter retrospective cohort study in Japan. Ann Surg Oncol. 2016;23:559–565. doi:10.1245/s10434-016-5380-3
52. Vitellaro M, Sala P, Signoroni S, et al. Risk of desmoid tumours after open and laparoscopic colectomy in patients with familial adenomatous polyposis. Br J Surg. 2014;101(5):558–565. doi:10.1002/bjs.9411
53. Ricciardiello L, Ahnen DJ, Lynch PM. Chemoprevention of hereditary colon cancers: time for new strategies. Nat Rev Gastroenterol Hepatol. 2016;13(6):352–361. doi:10.1038/nrgastro.2016.56
54. Burn J, Bishop DT, Chapman PD, et al. A randomized placebo-controlled prevention trial of aspirin and/or resistant starch in young people with familial adenomatous polyposis. Cancer Prev Res. 2011;4(5):655–665. doi:10.1158/1940-6207.CAPR-11-0106
55. Drew DA, Chan AT. Aspirin in the prevention of colorectal neoplasia. Annu Rev Med. 2021;72:415–430. doi:10.1146/annurev-med-060319
56. Ishikawa H, Mutoh M, Sato Y, et al. Chemoprevention with low-dose aspirin, mesalazine, or both in patients with familial adenomatous polyposis without previous colectomy (J-FAPP Study IV): a multicentre, double-blind, randomised, two-by-two factorial design trial. Lancet Gastroenterol Hepatol. 2021;6(6):474–481. doi:10.1016/S2468-1253(21)00018-2
57. Nanda N, Dhawan DK. Role of Cyclooxygenase-2 in Colorectal Cancer. Front Biosci. 2021;26(4):706–716.
58. Oshima M, Dinchuk JE, Kargman SL. Suppression of Intestinal Polyposis in Apc 716 Knockout Mice by Inhibition of CyclooxygenaseSuppression of intestinal polyposis in ApcΔ716 knockout mice by inhibition of cyclooxygenase 2 (COX-2). Cell. 1996;87(5):803–809. doi:10.1016/S0092-8674(00)81988-1
59. Cheng X, Xu X, Chen D, Zhao F, Wang W. Therapeutic potential of targeting the Wnt/β-catenin signaling pathway in colorectal cancer. Biomed Pharmacother. 2019;110:473–481. doi:10.1016/j.biopha.2018.11.082
60. Steinbach G, Lynch P, Phillips R, et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med. 1946;342(26):1946–1952. doi:10.1056/NEJM200006293422603
61. Ideon G, Teinbach S, Ynch AML, et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis a bstract background patients with familial adenomatous; 1946.
62. Lynch PM, Ayers GD, Hawk E, et al. The safety and efficacy of celecoxib in children with familial adenomatous polyposis. Am J Gastroenterol Suppl. 2010;105(6):1437–1443. doi:10.1038/ajg.2009.758
63. Yang P, Zuo X, Advani S, et al. Celecoxib colorectal bioavailability and chemopreventive response in patients with familial adenomatous polyposis. Cancer Prev Res. 2022;15(4):217–224. doi:10.1158/1940-6207.CAPR-21-0066
64. Bresalier RS, Sandler RS, Quan H, et al. Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N Engl J Med. 2005;352(11):1092–1102. doi:10.1056/NEJMoa050493
65. Baron JA, Sandler RS, Bresalier RS, et al. Cardiovascular events associated with rofecoxib: final analysis of the APPROVe trial. Lancet. 2008;372:1756–1764. doi:10.1016/S0140
66. Solomon SD, Wittes J, V. FP, et al. Cardiovascular risk of celecoxib in 6 randomized placebo-controlled trials: the cross trial safety analysis. Circulation. 2008;117(16):2104–2113. doi:10.1161/CIRCULATIONAHA.108.764530
67. Hall MJ. Updates in chemoprevention research for hereditary gastrointestinal and polyposis syndromes. Curr Treat Options Gastroenterol. 2021;19(1):30–46. doi:10.1007/s11938-020-00306-x
68. Ye SY, Li JY, Li TH, et al. The efficacy and safety of celecoxib in addition to standard cancer therapy: a systematic review and meta-analysis of randomized controlled trials. Curr Oncol. 2022;29(9):6137–6153. doi:10.3390/curroncol29090482
69. Giardiello FM, Hamilton SR, Krush AJ, et al. Treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. N Engl J Med. 1993;328(18):1313–1316. doi:10.1056/NEJM199305063281805
70. Giardiello F, Yang V, Hylind LM, et al. Primary chemoprevention of familial adenomatous polyposis with sulindac. N Engl J Med. 2002;346(14):1054–1059. doi:10.1056/NEJMoa012015
71. Moran AE, Hunt DH, Javid SH, Redston M, Carothers AM, Bertagnolli MM. Apc deficiency is associated with increased Egfr activity in the intestinal enterocytes and adenomas of C57BL/6J-Min/+ mice. J Biol Chem. 2004;279(41):43261–43272. doi:10.1074/jbc.M404276200
72. Baker H. Sulindac plus erlotinib for familial adenomatous polyposis. Lancet Oncol. 2016;17(5):e183. doi:10.1016/S1470-2045(16)30024-9
73. Delker DA, Wood AC, Snow AK, et al. Chemoprevention with cyclooxygenase and epidermal growth factor receptor inhibitors in familial adenomatous polyposis patients: mRNA signatures of duodenal Neoplasia. Cancer Prev Res. 2018;11(1):4–15. doi:10.1158/1940-6207.CAPR-17-0130
74. Samadder NJ, Neklason DW, Boucher KM, et al. Effect of sulindac and erlotinib vs placebo: on duodenal neoplasia in familial adenomatous polyposis: a randomized clinical trial. JAMA. 2016;315(12):1266–1275. doi:10.1001/jama.2016.2522
75. Samadder NJ, Kuwada SK, Boucher KM, et al. Association of sulindac and erlotinib vs placebo with colorectal neoplasia in familial adenomatous polyposis: secondary analysis of a randomized clinical trial. JAMA Oncol. 2018;4(5):671–677. doi:10.1001/jamaoncol.2017.5431
76. Samadder NJ, McMurray RP, Kanth P, et al. Phase II trial of weekly erlotinib dosing to reduce duodenal polyp burden associated with familial adenomatous polyposis. Gut. 2022;72:256–263. doi:10.1136/gutjnl-2021-326532
77. Meyskens FL, McLaren CE, Pelot D, et al. Difluoromethylornithine plus sulindac for the prevention of sporadic colorectal adenomas: a randomized placebo-controlled, double-blind trial. Cancer Prev Res. 2008;1(1):32–38. doi:10.1158/1940-6207.CAPR-08-0042
78. Burke CA, Dekker E, Lynch P, et al. Eflornithine plus sulindac for prevention of progression in familial adenomatous polyposis. N Engl J Med. 2020;383(11):1028–1039. doi:10.1056/nejmoa1916063
79. Balaguer F, Stoffel EM, Burke CA, et al. Combination of sulindac and eflornithine delays the need for lower gastrointestinal surgery in patients with familial adenomatous polyposis: post hoc analysis of a randomized clinical trial. Dis Colon Rectum. 2022;65(4):536–545. doi:10.1097/DCR.0000000000002095
80. Lynch PM, Burke CA, Phillips R, et al. An international randomised trial of celecoxib versus celecoxib plus difluoromethylornithine in patients with familial adenomatous polyposis. Gut. 2016;65(2):286–295. doi:10.1136/gutjnl-2014-307235
81. Hansen Petrik MB, Mcentee MF, Chiu CH, Whelan J. Biochemical and molecular action of nutrients antagonism of arachidonic acid is linked to the antitumorigenic effect of dietary eicosapentaenoic acid in apc min/ Mice 1; 2000. Available from: https://academic.oup.com/jn/article-abstract/130/5/1153/4686615. Accessed August 01, 2023.
82. West NJ, Clark SK, Phillips RKS, et al. Eicosapentaenoic acid reduces rectal polyp number and size in familial adenomatous polyposis. Gut. 2010;59(7):918–925. doi:10.1136/gut.2009.200642
83. Willenbacher E, Khan SZ, Mujica SCA, et al. Curcumin: new insights into an ancient ingredient against cancer. Int J Mol Sci. 2019;20(8):1808. doi:10.3390/ijms20081808
84. Cruz-Correa M, Shoskes DA, Sanchez P, et al. Combination treatment with curcumin and quercetin of adenomas in familial adenomatous polyposis. Clin Gastroenterol Hepatol. 2006;4(8):1035–1038. doi:10.1016/j.cgh.2006.03.020
85. Cruz-Correa M, Hylind LM, Marrero JH, et al. Efficacy and safety of curcumin in treatment of intestinal adenomas in patients with familial adenomatous polyposis. Gastroenterology. 2018;155(3):668–673. doi:10.1053/j.gastro.2018.05.031
86. Shenoy N, Creagan E, Witzig T, Levine M. Ascorbic acid in cancer treatment: let the phoenix fly. Cancer Cell. 2018;34(5):700–706. doi:10.1016/j.ccell.2018.07.014
87. Bussey HJ R, Decosse JJ, Deschner EE, et al. A randomized trial of ascorbic acid in polyposis coli. Cancer. 1982;50(7):1434–1439. doi:10.1002/1097-0142(19821001)50:73.0.CO;2-F
88. Decosse JJ, Miller HH, Lesser ML. ARTICLES effect of wheat fiber and vitamins C and E on rectal polyps in patients with familial adenomatous polyposis. JNCI. 1989;81(17):1290–1297. doi:10.1093/jnci/81.17.1290
89. Van Der Reest J, Gottlieb E. Anti-cancer effects of Vitamin C revisited. Cell Res. 2016;26(3):269–270. doi:10.1038/cr.2016.7
90. Yun J, Mullarky E, Lu C, et al. Vitamin C selectively kills KRAS and BRAF mutant colorectal cancer cells by targeting GAPDH. Science. 2015;350(6266):1391–1396. doi:10.1126/science.aaa5004
91. Rad E, Murray JT, Tee AR. Oncogenic signalling through mechanistic target of rapamycin (mTOR): a driver of metabolic transformation and cancer progression. Cancers. 2018;10(1):5. doi:10.3390/cancers10010005
92. Yuksekkaya H, Yucel A, Gumus M, Esen H, Toy H. Familial Adenomatous Polyposis; Successful Use of Sirolimus. Am J Gastroenterol Suppl. 2016;111(7):1040–1041. doi:10.1038/ajg.2016.159
93. Roos VH, Meijer BJ, Kallenberg FGJ, et al. Sirolimus for the treatment of polyposis of the rectal remnant and ileal pouch in four patients with familial adenomatous polyposis: a pilot study. BMJ Open Gastroenterol. 2020;7(1):e000497. doi:10.1136/bmjgast-2020-000497
94. Samadder NJ, Gornick M, Everett J, Greenson JK, Gruber SB. Inflammatory bowel disease and familial adenomatous polyposis. J Crohns Colitis. 2013;7(3):e103–e107. doi:10.1016/j.crohns.2012.06.021
95. Ishikawa H, Mutoh M, Abe T, et al. Utility of mesalazine in familial adenomatous polyposis: clinical report of reduction of polyp size in patients with ulcerative colitis, and safety examination in familial adenomatous polyposis patients. Pharmacology. 2019;104(1–2):51–56. doi:10.1159/000500226
96. Jung YS, Park CH, Eun CS, Park D, Han DS. Metformin use and the risk of colorectal adenoma: a systematic review and meta-analysis. J Gastroenterol Hepatol. 2017;32(5):957–965. doi:10.1111/jgh.13639
97. Zhang P, Li H, Tan X, Chen L, Wang S. Association of metformin use with cancer incidence and mortality: a meta-analysis. Cancer Epidemiol. 2013;37(3):207–218. doi:10.1016/j.canep.2012.12.009
98. Emami Riedmaier A, Fisel P, Nies AT, Schaeffeler E, Schwab M. Metformin and cancer: from the old medicine cabinet to pharmacological pitfalls and prospects. Trends Pharmacol Sci. 2013;34(2):126–135. doi:10.1016/j.tips.2012.11.005
99. Park JJ, Kim BC, Hong SP, et al. The effect of metformin in treatment of adenomas in patients with familial adenomatous polyposis. Cancer Prev Res. 2021;14(5):563–572. doi:10.1158/1940-6207.CAPR-20-0580