Infection
Microbiology and Clinical Outcome of Bloodstream Infections
1Center of Clinical Laboratory, The First Affiliated Hospital of Soochow University, Suzhou, Jiangsu, 215000, People’s Republic of China; 2Department of Infection Management, The First Affiliated Hospital of Soochow University, Suzhou, Jiangsu, 215000, People’s Republic of China
Purpose: Patients after hematopoietic stem cell transplantation (HSCT) are often followed by bloodstream infections (BSIs). BSI is an important cause of non-relapse mortality (NRM) in HSCT patients.
Methods: We conducted a retrospective cohort study of patients (aged > 14 years) who underwent HSCT at our hospital from 2017 to 2021. Population characteristics, BSI microbiology, resistance to common antibiotics, and 30-day all-cause mortality were analyzed.
Results: Of 3054 patients, 169 (5.5%) had BSIs after HSCT. Male, not in complete remission at transplantation and longer duration of neutropenia were risk factors for the development of BSI after HSCT. These BSIs were Gram-negative bacterial (n=123, 69.49%), Gram-positive bacterial (n=27, 15.25%), fungal (n=11, 6.36%), and polymicrobial (n=16, 9.25%). Among the Gram-negative bacteria, the proportions of isolates resistant to ceftazidime, cefepime, and piperacillin-tazobactam were similar (72.93%, 74.80%, and 77.42%, respectively). The overall drug resistance rates of amikacin and imipenem were 16.13% and 43.90%, respectively. Staphylococcus isolates were methicillin-resistant. In Enterococcus isolates, the penicillin resistance rate was 84.62%. Eleven isolates of Candida tropicalis were resistant to fluconazole and were sensitive to amphotericin B and flucytosine. The 30-day all-cause mortality rate of the 169 patients with BSIs was 8.88%. The 30-day all-cause mortality of patients with Gram-negative bacterial BSIs was 7.32%, 25.00% for polymicrobial BSIs, and 36.36% for fungal BSIs. The 30-day all-cause mortality of patients with fungal BSIs was significantly higher than that of patients with Gram-negative (P=0.0023) and Gram-positive bacteria (P=0.0023). Fungal BSI and non-Hodgkin’s lymphoma (NHL) were associated with higher 30-day mortality.
Conclusion: Our study reveals the microbiological characteristics and 30-day all-cause mortality in patients with bloodstream infections after HSCT. Our data provides strong support for empirical antimicrobial therapy and infection prevention strategies for patients with BSIs after HSCT.
Keywords: blood infections, hematopoietic stem cell transplantation, antibiotic resistance
Introduction
Hematopoietic stem cell transplantation (HSCT) is currently the most effective method for treating hematologic malignancies and non-malignant hematologic diseases. Infection is the most common complication of transplant failure and non-relapse mortality (NRM) in patients.1,2 Bloodstream infections (BSIs) often occur after HSCT and are associated with high morbidity and mortality.3–5 Literature reports that the incidence of BSIs in patients with HSCT ranges from 13.0% to 41.0%.6,7 Multiple single-center studies have shown that BSI within one year after HSCT is an important risk factor for early death in patients.8–10
Multidrug-resistant (MDR) and extensively drug-resistant (XDR) bacteria have become global concerns, posing significant challenges for patient treatment. Recent studies have reported an increasing incidence of BSIs in adult HSCT recipients caused by extended-spectrum beta-lactamase (ESBL), carbapenem-resistant Enterobacteriaceae (CRE), and vancomycin-resistant Enterococcus (VRE).11–13 The 28-day all-cause mortality rate of carbapenem-resistant Klebsiella pneumoniae is as high as 42.5%.14
Therefore, we investigated the pathogen spectrum, susceptibility to antibiotics, and 30-day all-cause mortality of patients with BSIs within one year after receiving HSCT at our hospital from 2017 to 2021. To understand the microbiology characteristics of BSIs and its clinical outcome in patients after HSCT, so as to guide the empirical prescription of antibiotics and infection prevention practice.
Materials and Methods
Study Design and Population
This was a retrospective, cohort study. Patients aged > 14 years who underwent HSCT at the First Affiliated Hospital of Soochow University between 2017 and 2021 were enrolled. Patients included 1758 males and 1296 females, with median age of 39 years (quartile range [IQR], 28–50 years). Of these, 1198 patients had acute myeloid leukemia (AML), 629 with acute lymphoblastic leukemia (ALL), 239 with non-Hodgkin’s lymphoma (NHL), 143 with aplastic anemia (AA), 333 with myelodysplastic syndromes (MDS), 286 with myeloma (MM) and 226 patients with other Hematological disease. Among 2523 patients of allogeneic hematopoietic stem cell transplantation (allo-HSCT), 502 were matched related transplantation, 1753 were mismatched related transplantation, and 261 were matched unrelated transplantation. According to the stem cell source, 1900 patients were received peripheral hematopoietic transplantation, 1145 were bone marrow transplantation, and nine were cord blood transplantation.
Conditioning Regimen and Prevention of Graft-versus-Host Disease (GVHD)
Patients with leukemia, lymphoma and myelodysplastic syndrome were treated with total body irradiation/cyclophosphamide (TBI/Cy) or modified busulfan/cyclophosphamide (Bu/Cy) or busulfan/fludarabine (Bu/Flu) conditioning regimen; Patients with aplastic anemia were treated with cyclophosphamide -anti-thymocyte globulin (Cy-ATG) pre-treatment regimen. Patients with multiple myeloma were treated with fludarabine/melphalan (Flu/Mel) or Mel regimen. The GVHD prevention program used cyclosporine combined with mycophenolate mofetil and methotrexate.
Anti-Infection Prophylaxis and Treatment Strategies
From pretreatment to ANC ≥ 1.0×109/L, patients routinely received anti-infection prophylaxis, with β-lactamases, and quinolones to prevent bacterial infections, and itraconazole to prevent fungal infections. After bloodstream infection occurs, the anti-infection treatment strategy should be adjusted based on the etiological diagnosis results, WBC, procalcitonin (PCT), 1,3-β-D glucan detection (G test), and galactomannitol glycan antigen detection (GM test).
Microbiological Assessment
The isolates were identified by VITEK-MS, and their antimicrobial susceptibility was determined using the VITEK-II system (bioMérieux, https://www.biomerieux.com), which was further confirmed using the broth micro-dilution method. The antifungal susceptibility of Candida isolates was determined using ATB Fungus 3 (bioMérieux, https://www.biomerieux.com). Antimicrobial and antifungal sensitivities were determined according to standards issued by the American Committee for Clinical and Laboratory Standardization (CLSI) and the European Committee for Antimicrobial Susceptibility Testing (EUCAST).
Definitions
Based on the National Healthcare Safety Network (NHSN) criteria, we defined BSIs as follows: (1) an identified pathogen growing from blood culture, or (2) a commensal organism growing from two blood cultures drawn from different sites at the same time or from the same site at different times. Within 14 days of the first positive blood culture, the growth of the same or different organisms obtained from the blood culture was considered to be from the same BSIs. We identified all BSIs that occurred in the study population within one year after HSCT. These BSIs are classified as Gram-negative bacterial, Gram-positive bacterial, fungal, or polymicrobial.
Statistical Analysis
Categorical data were presented as frequencies and proportions, continuous variables with normal distribution were reported as means and standard deviations (SDs), and non-normally distributed variables were expressed as medians and interquartile ranges (IQRs). Chi-square or Fisher’s exact test was used to compare categorical variables, and the Bonferroni method was used for multiple comparisons. The Student’s t-test or Mann–Whitney U-test was used to compare continuous variables. The Kruskal–Wallis H-test was used for multiple groups of non-normally distributed continuous variables and the Nemenyi method (R package PMCMRplus) was used for multiple comparisons. Variables with P values <0.1 in the univariate analyses were entered into logistic regression models for multivariate analysis to evaluate risk factors for BSI. The Kaplan-Meier product limit method was used to estimate the survival distribution function. The non-parametric Log rank test was used to compare the survival rates of each group. Cox proportional hazard regression model was adopted for multivariate analysis. Two-tailed P values <0.05 were considered statistically significant. Statistical analysis was performed using the SPSS version 23.0 software (SPSS Inc, Chicago, IL, USA) and R version 4.1.2.
Results
Patient Characteristics
The characteristics of the 3054 patients enrolled in this study were shown in Table 1. The median age of the 169 patients with BSIs after HSCT was 42 years (IQR, 29–51 years), and most patients (68.0%) were male. The median duration of neutropenia of the 169 patients was 16 days (IQR, 13–22 days). 88.2% of the 169 patients who developed BSIs after HSCT received allogeneic HSCT. Among the 169 patients, 80.5% received myeloablative conditioning, and 45.6% were not in complete remission at transplantation. 65.1% of the 169 patients received mismatched related transplantation, while peripheral blood was the most common source for HSCT in donors. AML, ALL, and MDS were the most common indicators of HSCT.
|
Table 1 Basic Characteristics of Patients Undergoing HSCT |
Risk Factors for the Development of BSI After HSCT
Univariate analysis results demonstrated that patients with male, AML, autologous HSCT (auto-HSCT), mismatched related transplantation, not in complete remission at transplantation and longer duration of neutropenia were more likely to develop BSI (Table 1). Meanwhile, multivariate analysis showed that male, not in complete remission at transplantation and longer duration of neutropenia were risk factors for the development of BSI after HSCT (Table 2).
 |
Table 2 Multivariate Analysis for Risk Factors for the Development of BSI After HSCT |
Characteristics of Bloodstream Infections
In our study, we identified 3054 patients who underwent HSCT, of whom 169 (5.5%) developed BSIs after HSCT. From 2017 to 2021, the annual incidence of BSIs was 2.6% (15/570), 4.7% (29/622), 5.9% (38/645), 6.8% (34/500), and 7.4% (53/717), respectively, with significant differences in the annual incidence (P= 0.002) (Figure 1). These BSIs were divided into Gram-negative bacterial (n = 123, 69.49%), Gram-positive bacterial (n = 27, 15.25%), fungal (n = 11, 6.36%), and polymicrobial (n = 16, 9.25%). We compared the time intervals between BSIs and HSCT in each group (Figure 2). The overall median time of onset of BSIs after HSCT was 7 (IQR, 2–9) days, 6 (IQR, 1–9) days for Gram-negative bacterial BSIs, 9 (IQR, 7.5–10) days for Gram-positive bacterial BSIs, 3 (IQR, 0.5–8.5) days for fungal BSIs; 7.5 (IQR, 1.75–10) days for polymicrobial BSIs. The time of BSIs after HSCT was significantly different among the different pathogen groups (P = 0.01). A post-hoc pair comparison among the groups showed that the time of BSIs relative to HSCT caused by Gram-negative bacteria was shorter than that caused by Gram-positive bacteria (P = 0.01).
|
Figure 1 The number of patients received HSCT and incidence of BSIs after HSCT in 2017–2021. |
|
Figure 2 The time of occurrence of BSIs in different groups relative to HSCT. |
Microbiology and Resistance to Antibiotics
A total of 177 strains were isolated from 169 BSIs (Table 3). Approximately 70% of the isolates were Gram-negative bacteria. Klebsiella pneumoniae (n=52), Escherichia coli (n=45), and Pseudomonas aeruginosa (n=10) were the most common Gram-negative bacteria. Gram-positive bacteria accounted for approximately one-fifth of cultured isolates. Enterococcus faecalis (n=10), Streptococcus mitis (n=6), and coagulase-negative Staphylococcus (CoNS) (n=5) were the most common Gram-positive bacteria. All fungal isolates were Candida, including Candida tropicalis (n=11) and Candida lusitaniae (n=1).
|
Table 3 Microbiology of Pathogens Causing BSIs After HSCT in 2017–2021 |
Table 4 summarized the antibiotic resistance of the pathogens that cause BSIs after HSCT. The antimicrobial susceptibility of specific pathogens to common antibiotics after HSCT was shown in Figure 3. Among Gram-negative bacteria, the proportions of resistance to ceftazidine, cefepime, and piperacillin-tazobactam were similar (72.93%, 74.80%, and 77.42%, respectively), while the proportion of resistance to ciprofloxacin was slightly higher (87.10%). The overall drug resistance rates of amikacin and imipenem were 16.13% and 43.90%, respectively. Among the 111 Enterobacteriaceae, 39.64% (n=44) were resistant to imipenem. Staphylococcus isolates were methicillin-resistant. In Enterococcus isolates, the penicillin resistance rate was 84.62%. Enterococcus faecalis isolates were resistant to penicillin. Eleven isolates of Candida tropicalis were resistant to fluconazole and were sensitive to amphotericin B and flucytosine. The Candida lusitaniae isolate was sensitive to fluconazole, amphotericin B, and flucytosine.
 |
Table 4 Antibiotic Resistance of Pathogens Causing BSIs After HSCT |
|
Figure 3 Antimicrobial susceptibility of specific pathogens to common antibiotics. (A) Klebsiella pneumoniae, n=52; (B) Escherichia coli, n=45; (C) Pseudomonas aeruginosa, n=10; (D) Enterococcus faecium, n=10. |
30-Day All-Cause Mortality and Risk Factors
We followed 169 patients with BSIs for 30 days, and the 30-day all-cause mortality rate was 8.88%. The 30-day all-cause mortality of patients with Gram-negative bacterial BSIs was 7.32%, 25.00% for polymicrobial BSIs, and 36.36% for fungal BSIs (Table 5). The 30-day all-cause mortality of BSIs patients in the different pathogen groups varied significantly (P<0.001) (Figure 4). The survival curves of the patients with pathogen-associated BSIs in each group were compared in pairs. The mortality of patients with fungal BSIs was significantly higher than that of patients with Gram-negative (P=0.0023) and Gram-positive bacteria (P=0.0023). According to multivariate analysis, Fungal BSI and NHL were significantly associated with higher 30-day all-cause mortality after BSI (Table 6).
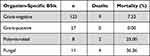 |
Table 5 Thirty-Day Mortality Associated with Organism-Specific BSIs (n =169) |
 |
Table 6 Univariate and Multivariate Analysis for Risk Factors for Thirty-Day Mortality |
|
Figure 4 Thirty-day survival curve of patients with different groups of BSIs. |
Discussion
In the post-transplant phase, owing to the presence of GVHD, especially in the intestinal tract, patients with secondary neutropenia are prone to BSIs.15 BSIs lead to increased length of hospital stay, intensive care stay, and mortality.1,8,16,17 In our cohort, 169 patients (5.5%) developed BSIs post-HSCT. Compared with other studies, we found a lower incidence of BSIs.1,16,18 This may be related to our clinical anti-infective prevention strategy.19
Previous studies have reported that the occurrence of BSIs in HSCT patients is associated with specific risk factors including severe GVHD,15,20 allogeneic HSCT,21,22 unrelated HSCT donors,23,24 intestinal colonization25 and myeloablative therapy.26 In our study, male, not in complete remission at transplantation and longer duration of neutropenia were risk factors for the development of BSI after HSCT.
In our study, 177 strains were isolated from 169 BSIs, of which 69.49% were Gram-negative bacteria. The most common bacterial strains were Klebsiella pneumoniae, Escherichia coli and Pseudomonas aeruginosa. Approximately half of Klebsiella pneumoniae isolates and one-fifth of Escherichia coli isolates were resistant to carbapenem. Sixty percent of Pseudomonas aeruginosa isolates were resistant to piperacillin-tazobactam and carbapenem. In recent years, the isolation rates of carbapenem-resistant Enterobacteriaceae (CRE) and multidrug-resistant Pseudomonas aeruginosa have gradually increased in patients undergoing HSCT,27–29 this brings great challenges to clinical treatment.6,27,30 Cao et al reported that forty-four HSCT recipients developed BSIs caused by CRE. The mortality rate of CRE-related BSIs within 30 days is as high as 50.0%.31 Another multicenter retrospective cohort study found that patients with malignant hematological diseases developed Pseudomonas aeruginosa BSIs, with a 30-day all-cause mortality rate of 37.3%.32 Enterococci were the most common Gram-positive bacteria isolated from BSIs in this study. Fortunately, Vancomycin-resistant Enterococci (VRE) were not detected. The 30-day mortality of VRE BSIs in HSCT patients is significantly higher than that of vancomycin-sensitive Enterococci BSIs.12,33
In addition, in our cohort, ten (5.92%) patients developed Candida tropicalis BSIs, one (0.59%) patient developed both Candida tropicalis and Klebsiella pneumoniae BSIs. Some studies in Spain have reported that 33 (2.8%) patients were diagnosed with fungal BSIs, and eight (12.3%) patients in southwestern China were diagnosed with fungal BSIs.6,27 In addition to fungal BSIs, researchers have reported concurrent CMV infection and fungemia,34 and mixed BSIs of bacteria and fungi.35 In our study, eleven Candida tropicalis isolates were resistant to fluconazole and were sensitive to amphotericin B and flucytosine. This may be related to the clinical use of itraconazole in the prevention of fungal infections.
In this study, 167 (98.8%) patients experienced BSIs within 30 days after HSCT. Heston et al reported that the median time of BSIs in 586 patients was 56 (IQR, 8–140.5) days after HSCT. This may be related to the different study populations; Heston’s study population included children less than 18 years old, and many patients had slow-growing non-tuberculosis mycobacteria in BSIs.7 However, this is consistent with our results showing that the time of BSIs relative to HSCT caused by Gram-negative bacteria was shorter than that caused by Gram-positive bacteria. Mikulska has also reported that in 149 patients who received HSCT, the median time to develop BSIs was 9 days after HSCT, and three-fourths of BSIs occurred within 20 days after HSCT.16
In this study, 169 patients with BSIs were followed for 30 days, and the 30-day all-cause mortality rate was 8.88%. Notably, the 30-day all-cause mortality of BSIs caused by fungi was as high as 36.36%, which was significantly higher than that of Gram-negative and Gram-positive bacteria. A multicenter retrospective study in Italy has found that 215 patients with malignant hematological diseases developed fungemia, and the mortality rates of mold and yeast infections are 70% and 39%, respectively.36 In eastern China, the 30-day all-cause mortality of 45 patients with malignant hematological diseases caused by Candida BSIs is 17.8%.35 In our study, we found fungal BSI and NHL were significantly associated with higher 30-day all-cause mortality after BSI. This reminds us to guard against the occurrence of fungal BSIs or fungal mixed BSIs in patients undergoing HSCT.
Our study has some limitations. First, this was a single-center retrospective cohort study that may not be generalizable to other institutions with different clinical practices. Second, due to the change in the method used by microbiology laboratories to report antibiotic sensitivity data, we were unable to determine the sensitivity of some bacteria to specific antibiotics. Finally, we did not define mucosal barrier damage-laboratory confirmed bloodstream infections.37,38 Therefore, we plan to collect multicenter data and conduct an in-depth analysis of the above factors in the future.
Conclusions
Our study reveals the microbiological characteristics of bloodstream infections after HSCT, the time of occurrence of BSIs relative to HSCT, and 30-day all-cause mortality. This study provides data to support empirical antimicrobial therapy and infection prevention strategies for patients with BSIs after HSCT.
Data Sharing Statement
The datasets used and/or analyzed during the present study are available from the corresponding author upon reasonable request.
Ethical Statement
This study was conducted according to the guidelines of the Declaration of Helsinki and was approved by the Institutional Review Committee of the First Affiliated Hospital of Soochow University (NO.2023-452). We confirmed that all data was anonymized and maintained with confidentiality; therefore, the requirement for informed consent was waived due to the retrospective design.
Funding
This work was supported by the Pfizer Foundation (Tracking No.76080151) and Key Project for Infection Management of the Suzhou Hospital Association (SZSYYXH-2023-ZD1).
Disclosure
The authors declare no competing interests in this work.
References
1. Poutsiaka DD, Price LL, Ucuzian A, Chan GW, Miller KB, Snydman DR. Blood stream infection after hematopoietic stem cell transplantation is associated with increased mortality. Bone Marrow Transplant. 2007;40(1):63–70. doi:10.1038/sj.bmt.1705690
2. Esquirol A, Pascual MJ, Kwon M, et al. Severe infections and infection-related mortality in a large series of haploidentical hematopoietic stem cell transplantation with post-transplant cyclophosphamide. Bone Marrow Transplant. 2021;56(10):2432–2444. doi:10.1038/s41409-021-01328-4
3. Willis DN, McGlynn MC, Reich PJ, Hayashi RJ. Mortality in pediatric oncology and stem cell transplant patients with bloodstream infections. Front Oncol. 2022;12:1063253. doi:10.3389/fonc.2022.1063253
4. Ohbayashi Y, Imataki O, Uemura M, et al. Oral microorganisms and bloodstream infection in allogeneic hematopoietic stem cell transplantation. Clin Oral Investig. 2021;25(7):4359–4367. doi:10.1007/s00784-020-03749-9
5. Akhmedov M, Klyasova G, Kuzmina L, Parovichnikova E. Recurrent bloodstream infections after allogeneic hematopoietic cell transplantation. Expert Rev Anti Infect Ther. 2023;21(1):87–90. doi:10.1080/14787210.2023.2151440
6. Zeng Q, Xiang B, Liu Z. Profile and antibiotic pattern of blood stream infections of patients receiving hematopoietic stem cell transplants in Southwest China. Infect Drug Resist. 2022;15:2045–2054. doi:10.2147/IDR.S358926
7. Heston SM, Young RR, Hong H, et al. Microbiology of bloodstream infections in children after hematopoietic stem cell transplantation: a single-center experience over two decades (1997–2017). Open Forum Infect Dis. 2020;7(11):ofaa465. doi:10.1093/ofid/ofaa465
8. Ustun C, Young JH, Papanicolaou GA, et al. Bacterial blood stream infections (BSIs), particularly post-engraftment BSIs, are associated with increased mortality after allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2019;54(8):1254–1265. doi:10.1038/s41409-018-0401-4
9. Yan CH, Wang Y, Mo XD, et al. Incidence, risk factors, microbiology and outcomes of pre-engraftment bloodstream infection after haploidentical hematopoietic stem cell transplantation and comparison with HLA-identical sibling transplantation. Clin Infect Dis. 2018;67(suppl_2):S162–S173. doi:10.1093/cid/ciy658
10. Gill J, Busca A, Cinatti N, et al. Bacterial bloodstream infections after allogeneic hematopoietic stem cell transplantation: etiology, risk factors and outcome in a single-center study. Microorganisms. 2023;11(3):742. doi:10.3390/microorganisms11030742
11. Ferreira AM, Moreira F, Guimaraes T, et al. Epidemiology, risk factors and outcomes of multi-drug-resistant bloodstream infections in haematopoietic stem cell transplant recipients: importance of previous gut colonization. J Hosp Infect. 2018;100(1):83–91. doi:10.1016/j.jhin.2018.03.004
12. Papanicolaou GA, Ustun C, Young JH, et al. Bloodstream infection due to vancomycin-resistant enterococcus is associated with increased mortality after hematopoietic cell transplantation for acute leukemia and myelodysplastic syndrome: a multicenter, retrospective cohort study. Clin Infect Dis. 2019;69(10):1771–1779. doi:10.1093/cid/ciz031
13. Bes T, Nagano D, Martins R, et al. Bloodstream infections caused by Klebsiella pneumoniae and Serratia marcescens isolates co-harboring NDM-1 and KPC-2. Ann Clin Microbiol Antimicrob. 2021;20(1):57. doi:10.1186/s12941-021-00464-5
14. Chen J, Ma H, Huang X, et al. Risk factors and mortality of carbapenem-resistant Klebsiella pneumoniae bloodstream infection in a tertiary-care hospital in China: an eight-year retrospective study. Antimicrob Resist Infect Control. 2022;11(1):161. doi:10.1186/s13756-022-01204-w
15. Mori Y, Yoshimoto G, Nishida R, et al. Gastrointestinal graft-versus-host disease is a risk factor for postengraftment bloodstream infection in allogeneic hematopoietic stem cell transplant recipients. Biol Blood Marrow Transplant. 2018;24(11):2302–2309. doi:10.1016/j.bbmt.2018.06.002
16. Mikulska M, Del Bono V, Bruzzi P, et al. Mortality after bloodstream infections in allogeneic haematopoietic stem cell transplant (HSCT) recipients. Infection. 2012;40(3):271–278. doi:10.1007/s15010-011-0229-y
17. Zhu Q, Xu J, Chen X, Ren Y, Zhao L. Risk factors and molecular epidemiology of bloodstream infections due to carbapenem-resistant Klebsiella pneumoniae. Diagn Microbiol Infect Dis. 2023;106(3):115955. doi:10.1016/j.diagmicrobio.2023.115955
18. Chen W, Zhao Y, Luo Y, et al. Clinical characteristics, microbiology, and risk factors for mortality of pre-engraftment and post-engraftment bloodstream infection in hematopoietic stem cell transplantation recipients. Infect Drug Resist. 2022;15:6893–6905. doi:10.2147/IDR.S392804
19. Han QZ, Chen Y, Yang H, et al. 血流感染在1265例造血干细胞移植患者中的发生情况及病原菌分析 [Incidence of blood stream infections of 1265 patients with hematopoietic stem cell transplantation and analysis of pathogenic bacteria]. Zhonghua Xue Ye Xue Za Zhi. 2017;38(11):930–933. Chinese. doi:10.3760/cma.j.issn.0253-2727.2017.11.005
20. Poutsiaka DD, Munson D, Price LL, Chan GW, Snydman DR. Blood stream infection (BSI) and acute GVHD after hematopoietic SCT (HSCT) are associated. Bone Marrow Transplant. 2011;46(2):300–307. doi:10.1038/bmt.2010.112
21. Hong J, Moon SM, Ahn HK, et al. Comparison of characteristics of bacterial bloodstream infection between adult patients with allogeneic and autologous hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2013;19(6):994–999. doi:10.1016/j.bbmt.2013.03.019
22. Girmenia C, Bertaina A, Piciocchi A, et al. Incidence, risk factors and outcome of pre-engraftment gram-negative bacteremia after allogeneic and autologous hematopoietic stem cell transplantation: an Italian Prospective Multicenter Survey. Clin Infect Dis. 2017;65(11):1884–1896. doi:10.1093/cid/cix690
23. Young JH, Logan BR, Wu J, et al. Infections after transplantation of bone marrow or peripheral blood stem cells from unrelated donors. Biol Blood Marrow Transplant. 2016;22(2):359–370. doi:10.1016/j.bbmt.2015.09.013
24. Takagi S, Ogura S, Araoka H, et al. The impact of graft cell source on bloodstream infection in the first 100 days after allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2021;56(7):1625–1634. doi:10.1038/s41409-021-01229-6
25. Forcina A, Lorentino F, Marasco V, et al. Clinical impact of pretransplant multidrug-resistant gram-negative colonization in autologous and allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2018;24(7):1476–1482. doi:10.1016/j.bbmt.2018.02.021
26. Akinboyo IC, Young RR, Spees LP, et al. Microbiology and risk factors for hospital-associated bloodstream infections among pediatric hematopoietic stem cell transplant recipients. Open Forum Infect Dis. 2020;7(4):ofaa093. doi:10.1093/ofid/ofaa093
27. Puerta-Alcalde P, Cardozo C, Marco F, et al. Changing epidemiology of bloodstream infection in a 25-years hematopoietic stem cell transplant program: current challenges and pitfalls on empiric antibiotic treatment impacting outcomes. Bone Marrow Transplant. 2020;55(3):603–612. doi:10.1038/s41409-019-0701-3
28. Cao W, Guan L, Li X, et al. Clinical analysis of bloodstream infections during agranulocytosis after allogeneic hematopoietic stem cell transplantation. Infect Drug Resist. 2021;14:185–192. doi:10.2147/IDR.S280869
29. Castagnola E, Bagnasco F, Mesini A, et al. Antibiotic resistant bloodstream infections in pediatric patients receiving chemotherapy or hematopoietic stem cell transplant: factors associated with development of resistance, intensive care admission and mortality. Antibiotics. 2021;10(3):266. doi:10.3390/antibiotics10030266
30. Trecarichi EM, Pagano L, Martino B, et al. Bloodstream infections caused by Klebsiella pneumoniae in onco-hematological patients: clinical impact of carbapenem resistance in a multicentre prospective survey. Am J Hematol. 2016;91(11):1076–1081. doi:10.1002/ajh.24489
31. Cao W, Zhang J, Bian Z, et al. Active screening of intestinal colonization of carbapenem-resistant Enterobacteriaceae for subsequent bloodstream infection in allogeneic hematopoietic stem cell transplantation. Infect Drug Resist. 2022;15:5993–6006. doi:10.2147/IDR.S387615
32. Royo-Cebrecos C, Laporte-Amargos J, Pena M, et al. Pseudomonas aeruginosa bloodstream infections in patients with cancer: differences between patients with hematological malignancies and solid tumors. Pathogens. 2022;11(10):1132. doi:10.3390/pathogens11101132
33. Vydra J, Shanley RM, George I, et al. Enterococcal bacteremia is associated with increased risk of mortality in recipients of allogeneic hematopoietic stem cell transplantation. Clin Infect Dis. 2012;55(6):764–770. doi:10.1093/cid/cis550
34. Öksüz L, Hindilerden I Y, Erciyestepe M, et al. The association of CMV infection with bacterial and fungal infections in hematopoietic stem cell transplant recipients: a retrospective single-center study. New Microbiol. 2022;45(1):40–50.
35. Chen X-C, Xu J, Wu D-P. Clinical characteristics and implications of mixed candida/bacterial bloodstream infections in patients with hematological diseases. Eur J Clin Microbiol Infect Dis. 2020;39(8):1445–1452. doi:10.1007/s10096-020-03863-2
36. Criscuolo M, Marchesi F, Candoni A, et al. Fungaemia in haematological malignancies: SEIFEM-2015 survey. Eur J Clin Invest. 2019;49(5):e13083. doi:10.1111/eci.13083
37. Dandoy CE, Kim S, Chen M, et al. Incidence, risk factors, and outcomes of patients who develop mucosal barrier injury-laboratory confirmed bloodstream infections in the first 100 days after allogeneic hematopoietic stem cell transplant. JAMA Netw Open. 2020;3(1):e1918668. doi:10.1001/jamanetworkopen.2019.18668
38. Dandoy CE, Haslam D, Lane A, et al. Healthcare burden, risk factors, and outcomes of mucosal barrier injury laboratory-confirmed bloodstream infections after stem cell transplantation. Biol Blood Marrow Transplant. 2016;22(9):1671–1677. doi:10.1016/j.bbmt.2016.06.002