Cardiovascular
Almost 100 Million Americans Eligible For New Weight Loss Drug
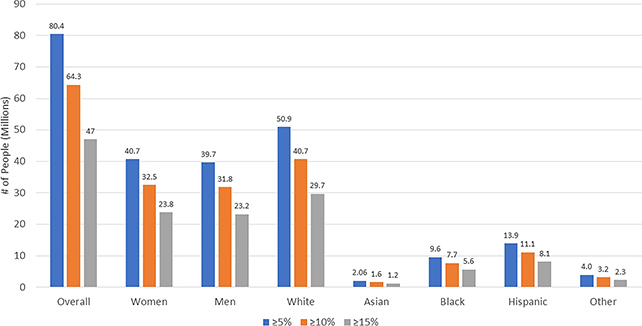
A diabetes drug distributed under the brand name Wegovy could offer a suitable and beneficial weight loss treatment for some 93 million adults in the US, according to a new study.
That’s a lot of people by any measure. Projections suggest it would mean some 43 million fewer people with obesity, and the prevention of around 1.5 million heart attacks, strokes, and other cardiovascular problems over the course of 10 years.
The estimates, from a team at the University of California, Irvine (UCI) in the US, are based on a recent trial involving 1,961 adults, targeted at people with obesity and who were given a weekly 2.4 milligram dose of semaglutide, the pharmaceutical on which Wegovy is based.
Trialing a dosage already approved by the US Food and Drug Administration (FDA), the effects were dramatic: an average body weight reduction of 14.9 percent, and a reduction in risk factors associated with cardiovascular problems, including blood pressure and fatty compounds called lipids.
“It is one of the biggest advances in the obesity and cardiovascular medicine world,” says Nathan Wong, a professor of medicine at UCI.
“We now have a weight control therapy that also significantly reduces cardiovascular events beyond the diabetes population where it was originally studied.”
Semaglutide works by mimicking the GLP-1 hormone released naturally by our gut as we eat. One of the hormone’s jobs is to increase production of insulin and thus reduce blood sugar (glucose) levels, which is why it’s previously been used to treat diabetes.
However, scientists have also discovered that it targets the parts of our brains that tell us we’re full, suppressing appetite. This can lead to significant weight loss – as long as it’s combined with a managed diet and exercise.
The researchers came up with their 93 million figure by extrapolating the criteria for the previous trial to the broader population, but they emphasize that no drug should be taken without a consultation with a doctor.
Right now semaglutide is approved for use for chronic weight management in adults who are obese or overweight, and who also have at least one weight-related condition – such as high blood pressure, type 2 diabetes, or high cholesterol.
A broader roll-out of the drug and others like it could meet a rising need: by 2030, half of US adults are expected to be considered obese with nearly 1 in 4 falling into a severely obese category. This puts many at greater risk of a wealth of health problems, including heart disease and heart failure.
“It should be considered for patients who are obese or overweight with other risk factors where cardiovascular disease is their leading cause of disability and death,” says Wong.
The research has been published in Cardiovascular Drugs and Therapy.