Congenital disorders
Valproate Prescriptions to Reproductive-Age Women in Ambulatory Care
This work may not be copied, distributed, displayed, published, reproduced, transmitted, modified, posted, sold, licensed, or used for commercial purposes. By downloading this file, you are agreeing to the publisher’s Terms & Conditions.
Published: August 22, 2023
Reproductive-age women exposed to valproate have an increased risk of polycystic ovarian syndrome, which can cause pelvic pain, abnormal periods, hirsutism, acne, and infertility.1 In addition, valproate is a human teratogen, and exposure during pregnancy was shown to increase the risk of congenital malformations 5-fold compared to controls.2,3 Valproate products comprise sodium valproate, divalproex sodium, valproic acid, and other generics (hereafter collectively called valproate). With the increased awareness about its teratogenicity, several countries have decreased prescriptions of valproate to reproductive-age women.4,5 In the United States, 45% of pregnancies are unintended, and several states restrict elective abortion, making it more critical to decrease valproate prescriptions.6,7 However, in the last 10 years, no study has been done on the latest trend in valproate prescription for reproductive-age women. Therefore, this study aims to fill this gap in the literature.
METHODS
This study was a secondary analysis of publicly available de-identified data from the National Ambulatory Medical Care Survey (NAMCS) and the National Hospital Ambulatory Medical Care Survey (NHAMCS) for 2018 and 2019.8 NAMCS data are obtained from a national sample of office-based physician visits, while NHAMCS contains data on visits to hospital emergency departments in the United States. Multum Lexicon (Cerner Multum, Inc) third-level therapeutic category codes were used to define valproate (code 345). Survey data were analyzed using the sampled visit weight, adjusted to yield an unbiased national estimate. Because of the complex sample design, sampling errors were determined using IBM SPSS statistics version 28 with a complex sampling module, which considers the clustered nature of the sample.
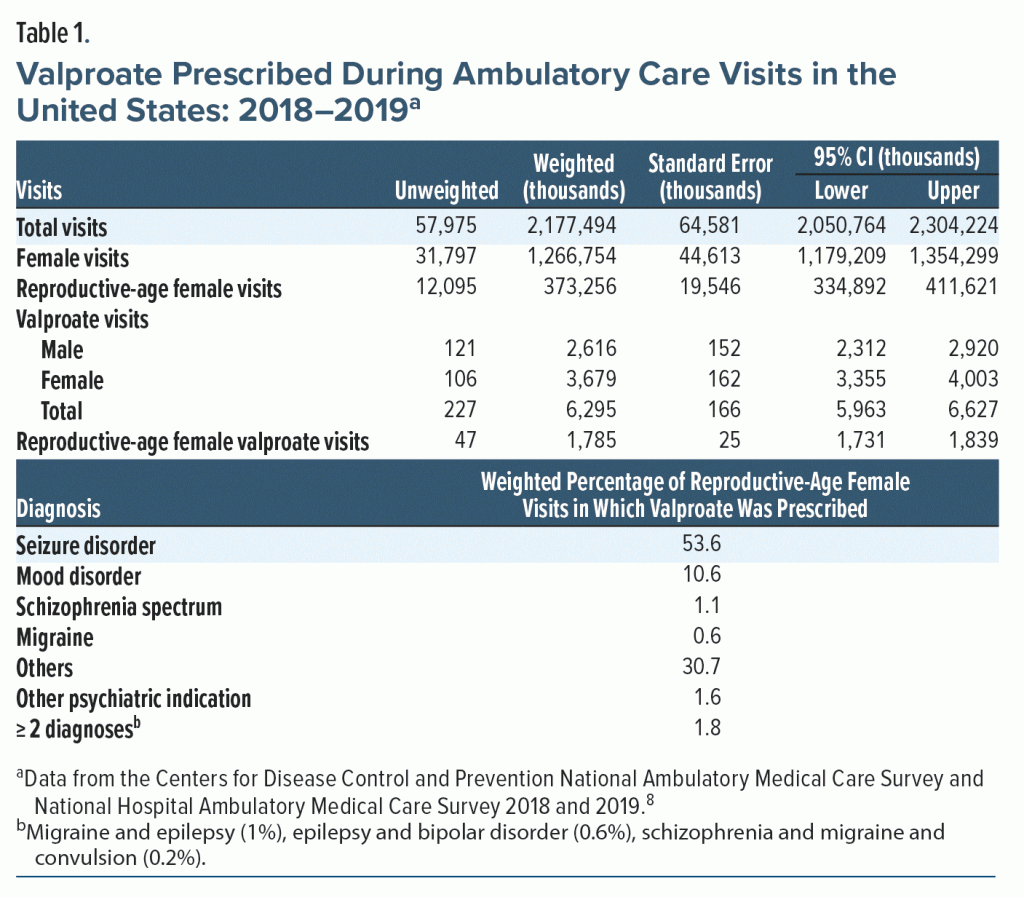
Visits of reproductive-age women aged 15–44 years in which valproate was prescribed were extracted and further grouped using ICD-10–CM codes as follows: (1) seizure disorders, (2) mood disorders, (3) schizophrenia and other psychosis, (4) migraine/headache, (5) other psychiatric indications, (6), and others (see Supplementary Table 1 for a list of ICD-10-CM codes). Since the dataset is de-identified and publicly available, the study was exempt from review by the institutional review board. The data analysis was conducted in the United States.
RESULT
Valproate was prescribed more during female visits than in male visits (3.68 million vs 2.62 million). Of the 1.7 billion female visits, 373 million (29%) were of reproductive age (aged 15–44 years). Valproate was prescribed in 1.78 million reproductive-age female visits (4.8 visits, 95% CI, 4.5–5.2 per 1,000 reproductive-age female visits). Of those visits, 53.6% had a seizure disorder and 10.6% had a mood disorder, while in 30.7% of visits, there was a diagnosis for which valproate does not have a US Food and Drug Administration–approved or common off-label use (Table 1).

DISCUSSION
Prescription of valproate to reproductive-age women in 2018–2019 in ambulatory care across the United States remained unchanged compared to the study by Adedinsewo et al2 10 years prior, which found 4.45 visits (95% CI, 3.8–5.06).
The prescription of valproate has decreased for psychiatric diagnoses and increased for seizure diagnoses. Of total valproate visits between 1996 and 2007, 61% had a psychiatric diagnosis, and 17% had a seizure diagnosis.2 In our study, these rates changed to 14% for psychiatric disorders and 54% for seizure disorders. The decrease in valproate prescriptions for psychiatric diagnosis is possibly due to second-generation antipsychotics being preferred over mood stabilizers for mood disorders over the last decade.9
Interestingly, for about 30% of the visits, there was no matching diagnosis for which valproate is FDA approved or has common off-label use. Since the survey’s primary focus is on the content of the individual visit, chronic diseases may have not been accurately recorded. However, given the teratogenic potential of valproate, these are concerning findings that deserve further study.
CONCLUSION
The prescription of valproate in reproductive-age female visits in ambulatory care in the last decade has remained unchanged. There was a decrease in valproate prescribed for visits with psychiatric disorders but an increase for seizure disorders.
Article Information
Published Online: August 22, 2023. https://doi.org/10.4088/PCC.22br03500
© 2023 Physicians Postgraduate Press, Inc.
Prim Care Companion CNS Disord. 2023;25(4):22br03500
Submitted: January 28, 2023; accepted April 4, 2023.
To Cite: Rizvi A, Shaan F, Reyazuddin M. Valproate prescribed to reproductive age women in ambulatory care: analysis of 2018 and 2019 national ambulatory health care data. Prim Care Companion CNS Disord. 2023;25(4):22br03500.
Author Affiliations: Department of Behavioral Medicine and Psychiatry, West Virginia University, Morgantown (Rizvi); Department of Psychiatry, Aligarh Muslim University, Uttar Pradesh, India (Shaan, Reyazuddin).
Corresponding Author: Abid Rizvi, MD, 936 Sharpe Hospital Rd, Weston, West Virginia 26452([email protected]).
Relevant Financial Relationships: None.
Funding/Support: None.
Supplementary Material: See accompanying pages.