Congenital disorders
How Neurons Grow Comfortable in Their Own Skin
At a glance:
- Neurons that sense different types of touch are “born” unspecialized but develop specific features based on the skin type they end up innervating.
- The finding, based on research in mice, sheds light on a long-standing mystery about how these nerve cells end up performing their specialized tasks.
- The research could eventually help scientists regenerate damaged or diseased nerves, understand certain inherited nerve disorders.
Nerve cells that sense touch grow the appropriate endings for hairy or hairless skin based on cues from the skin itself, rather than through predetermined programming, according to research led by Harvard Medical School scientists and published Aug. 21 in Developmental Cell.
If affirmed in further studies, the findings could eventually help researchers develop therapies to regenerate damaged or diseased nerves, the authors say, or better understand what goes awry in congenital neuropathies, conditions in individuals born with sensory nerve defects.
Get more HMS news here
“The take-home message is that it’s the skin that tells unspecified neurons how to mature in shape, size, and structure to become the ending type appropriate for that specific skin region,” said study senior author David Ginty, chair of the Department of Neurobiology at the Blavatnik Institute at HMS and a Howard Hughes Medical Institute investigator. The study was led by Charalampia Koutsioumpa, a former graduate student in the Ginty Lab who is now a neurology resident at Yale Medicine.
About 20 or so different types of neurons innervate the skin to detect a wide range of sensations, such as temperature, pain, or touch, Ginty explained. During development, these nerve cells send extensions, called axons, from cell bodies located near the spinal cord to the skin.
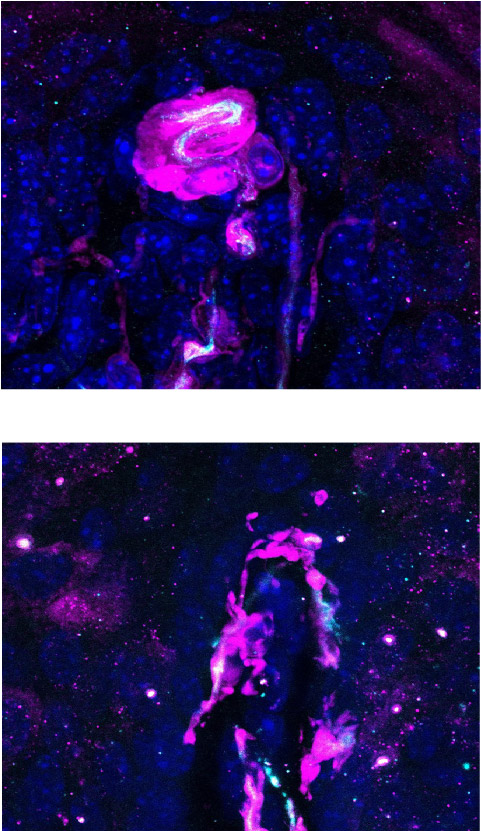
When they reach their final destinations in the skin, the axons form so-called terminations, known as end organs, which are tailored to the neurons’ functions. For example, neurons that sense light touch (called low-threshold mechanoreceptors, or LTMRs) have end organs called Meissner corpuscles if they terminate in hairless skin, such as on the palms of the hands, giving them relatively high sensing acuity.
In contrast, those that end up in hairy skin, which covers more than 90 percent of the body surface, have end organs called lanceolate complexes with lower sensing acuity but capable of detecting nonspecific stimuli, such as touching the skin or wind blowing through hair.
Although researchers have known about these different sensory nerve types for decades, said Koutsioumpa, it has remained unclear how they arise to become so specialized.
Two theories have floated around: Either these nerve endings have a set fate when they’re generated, sending projections to their predetermined destination of hairless or hairy skin, or they arise unspecialized, developing into one type (the highly sensitive Meissner corpuscles) or the other (the less sensitive lanceolate complexes), based on cues from the skin itself.
To determine which of these theories was correct, Koutsioumpa, Ginty, and their colleagues used mice as a model system. These animals have hairless skin on their paw pads analogous to that on humans’ palms and soles and hairy skin that covers most of the rest of their bodies.
Using a genetic labeling technique, the researchers generated mice with sensory neurons that could be seen in the skin, allowing them to track the growth of these cells starting before birth. Although the different nerve endings in hairless and hairy skin became distinguishable within a week after birth, these neurons looked identical in utero, which meant that their differences arise later in development. This finding gave the first clue that development of the highly sensitive Meissner corpuscles, or the less sensitive lanceolate end organs, is not predetermined but might be dictated by final-destination skin type, Koutsioumpa said.
Over the course of this experiment, the researchers spotted some individual sensory neurons that branched into multiple endings at border regions between hairless and hairy skin. Although these endings sprang from the same cell body, they developed Meissner corpuscles and lanceolate end organs depending on whether their endings were in hairless or hairy territory. Again, this finding supported the notion that end organs developed based on signals from the skin the neurons ended up innervating. In other words, the shape, size, structure — and ultimately fate — of these neurons were modulated by cues from the skin type they served.
In another experiment, the researchers worked with mice carrying a mutation that gave them hairless skin not only on their paw pads, but on the tops of their paws as well. Although this mutation presumably affected just the skin itself, an examination of the sensory neurons innervating the paw tops showed that they developed highly sensitive Meissner corpuscles, just like those in the paw pad skin, suggesting that a signal from the skin had influenced neural type development.
When the researchers searched for molecules that might serve as that signal, previous research and their own experiments suggested two candidates, known as bone morphogenic proteins 5 and 7 (BMP5 and BMP7), that were heavily expressed in hairless skin.
Sure enough, when the researchers genetically altered mice to remove the receptors for this family of proteins, their hairless skin had far fewer Meissner corpuscles, and those that remained developed abnormally. Although BMP5 and BMP7 appear to be key for Meissner corpuscle development, the researchers did not identify any candidate signal molecules for lanceolate complexes.
Together, Koutsioumpa said, these findings suggest that skin type determines which end organs sensory neurons will develop, a finding that could influence efforts to regenerate sensory neurons in the skin. It could also eventually help researchers develop treatments for sensory neuropathies, one of the conditions that she plans to treat as a neurologist.
“Sensory neuropathies are some of the most common inherited neurological disorders,” Koutsioumpa said. “Our work might give some clues to therapies in the future.”
Authorship, funding, disclosures
Additional authors include Celine Santiago, Kiani Jacobs, Brendan P. Lehnert, Victor Barrera, John N. Hutchinson, Dhane Schmelyun, Jessica A. Lehoczky, and David L. Paul.
This work was funded by a Boehringer Ingelheim Fonds fellowship, an Onassis Scholarship, the Harvard Medical School Foundry Program, a grant from the National Institutes of Health (R35 5R35NS097344-05).