Cardiovascular
Early elevation of high-sensitivity C-reactive protein as a predictor for cardiovascular disease incidence and all-cause mortality: a landmark analysis
Abstract
We investigated the association between early elevation of high-sensitivity C-reactive protein (hsCRP) and cardiovascular disease (CVD) incidence, all-cause mortality, and CVD mortality. We analyzed 6567 participants from the Korean Genome and Epidemiology Study_Ansan_Ansung cohort between 2005 and 2018. The Kaplan–Meier curves and modified Cox regression by Fine and Gray were used to estimate hazard ratios (HRs) with 95% confidence intervals (CIs) for CVD incidence, all-cause mortality, CVD mortality, cancer mortality, and mortality from other causes. Landmark analyses were performed at the first (2007–2008) and second (2009–2010) follow-up periods, with early elevation defined as hsCRP > 2 mg/L. At the first and second landmark points, the early hsCRP elevation group had a higher incidence of CVD and all-cause mortality. At first landmark point, the adjusted HRs (95% CIs) were 1.37 (1.08–1.74) for incident CVD and 1.26 (1.04–1.53) for all-cause mortality, respectively. At second landmark point, the adjusted HRs in the early hsCRP elevation group were 1.45 (1.12–1.89) for incident CVD and 1.34 (1.10–1.63) for all-cause mortality, respectively. However, there were no significant differences in CVD mortality and cancer mortality between the groups. In conclusion, early elevation of serum hsCRP is a predictor of incident CVD and all-cause mortality. The timing of hsCRP increase is also a significant predictor of incident CVD, even considering the competing risk. Regular hsCRP testing may help monitor hsCRP trends and develop individualized treatment plans for CVD prevention.
Introduction
Cardiovascular disease (CVD) is a major global health concern, which is responsible for the highest number of deaths worldwide. According to the results from global burden of disease study, CVD mortality steadily increased from 12.1 million in 1990 to 18.6 million in 2019 and accounted for 31.59% of all deaths in 20171,2,3. Moreover, the number of incident cases of CVD increased by 77.12% between 1990 and 2019, rising from 31.31 million to 55.45 million3. Atherosclerosis, a major cause of CVD, is a chronic inflammatory condition that causes plaque buildup in artery walls, resulting in narrowed and hardened blood vessels4,5. Various inflammatory markers, such as high-sensitivity C-reactive protein (hsCRP), interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-α), have been used to assess atherosclerosis risk and progression, in addition to traditional risk factors, such as cigarette smoking, hypertension (HTN), dyslipidemia, and diabetes mellitus (DM)5,6,7,8,9.
Serum hsCRP is one of the most widely used inflammatory biomarkers for atherosclerosis risk assessment in clinical practice9,10. Although there is firm evidence on the association of elevated serum hsCRP with the risk of CVD, most of previous studies have evaluated the risk of CVD development based on the cross-sectional hsCRP status11,12,13. However, cumulative exposure to elevated hsCRP also can predict the risk of CVD14. Wang et al.14 found that the risk of incident CVD was elevated in dose–response manner with the cumulative number of elevated hsCRP event. In this regard, there is possibility that serum hsCRP levels increase above a certain threshold over shorter time intervals also could be a predictor for the risk of CVD. There is evidence showing the impact of early elevation of inflammatory markers on adverse clinical outcomes15,16. Emsley et al.15 found that 6 patients with acute ischemic stroke exhibited early and sustained peripheral inflammatory responses, including elevations in CRP, erythrocyte sedimentation rate, and white blood cell count, particularly in the first 5–7 days after onset of symptoms, compared to 36 controls. These findings suggest that preexisting inflammatory conditions may contribute to stroke development. Additionally, Cuschieri et al.16 reported that early elevation in plasma IL-6 levels following severe injury was correlated with incident organ failure among 79 severely injured patients with shock. These findings suggest the importance of timing with regard to the elevation of inflammatory markers. From this perspective, we hypothesized that early elevation of hsCRP levels may also indicate a higher risk of developing CVD. Despite this assumption, there is currently limited evidence regarding the association between early hsCRP elevation and CVD risk.
Given that serum hsCRP levels can be monitored over time in clinical practice, clinicians may be able to reduce the risk of CVD by implementing early, intensive management of cardiovascular risk factors in individuals who exhibit early hsCRP elevation, given that it is associated with a higher risk of CVD incidence and mortality. Therefore, this study aimed to investigate whether early elevation of serum hsCRP level is associated with an increased risk of CVD incidence and mortality, using landmark analysis in a community-based, prospective cohort study.
Results
Baseline characteristics of the study population
Table 1 presents the baseline characteristics of the study population stratified by hsCRP status at different time points. In the full dataset, participants with at least one hsCRP > 2 mg/L event during the follow-up were more likely to be men, older, and have higher body mass index (BMI), waist circumference (WC), mean blood pressure (MBP), fasting plasma glucose (FPG), triglycerides, and prevalence of DM, HTN, and dyslipidemia and lower high-density lipoprotein (HDL) cholesterol than those with consistently hsCRP ≤ 2 mg/L. There were no significant differences in total energy intake, drinking status, or exercise status between the two groups. Similar trends were observed at both the first and second follow-up landmark points, except for serum low-density lipoprotein (LDL) cholesterol, which did not show a significant difference between the groups, with and without early hsCRP elevation.
Longitudinal association of elevation of hsCRP with incident CVD and all-cause mortality at different time points
During the median 11.84 years of the event accrual period, there were a total of 441 (6.72%) new-onset CVD cases. The CVD incidence rate per 1000 person-years was 6.05 in the group with hsCRP > 2 mg/L during the follow-up and 3.55 in the group with consistent hsCRP ≤ 2 mg/L. At the first landmark point, the CVD incidence rate per 1000 person-years was 5.50 in the group with early elevation of hsCRP and 3.36 in the group without early elevation. At the second landmark point, the CVD incidence rate per 1000 person-years was 4.42 in the group with early elevation of hsCRP and 2.60 in the group without early elevation. During the median 14.64 years of event accrual period, a total of 581 (8.85%) all-cause mortality cases and 114 CVD mortality cases were observed. The all-cause mortality rate per 1000 person-years was 6.90 in the group with hsCRP > 2 mg/L events at least once during the follow-up and 4.69 in the group with consistently hsCRP ≤ 2 mg/L. At the first landmark point, the all-cause mortality rate per 1000 person-years was 8.16 in the group with early elevation of hsCRP and 4.79 in the group without early elevation. At the second landmark point, the all-cause mortality rate per 1000 person-years was 7.87 in the group with early elevation of hsCRP and 4.30 in the group without early elevation.
Figure 1A–F show the Kaplan–Meier curves with log-rank tests for the cumulative incidence of CVD according to hsCRP elevation events at different time points. The group with hsCRP > 2 mg/L events at least once during follow-up had significantly higher cumulative rates of incident CVD than the group with consistently hsCRP < 2 mg/L (log-rank test p < 0.001 for all datasets; Fig. 1A–C). The same trends were observed for all-cause mortality (log-rank test p < 0.001 for all datasets; Fig. 1D–F).
Kaplan–Meier curves showing the cumulative incidence rate of CVD and all-cause mortality according to the hsCRP elevation event at different time points, including full dataset (A incident CVD, D all-cause mortality), landmark at 1st follow-up (B incident CVD, E all-cause mortality), and landmark at 2nd follow-up: all-cause mortality (C incident CVD, F all-cause mortality). hsCRP high-sensitivity C-reactive protein, CVD cardiovascular disease.
Table 2 presents the results of modified Cox regression by Fine and Gray, examining the association between elevated serum hsCRP levels and incident CVD and all-cause mortality at different time points. For the full dataset, the group with hsCRP > 2 mg/L event at least once during follow-up period had a higher risk of incident CVD than the group with consistently hsCRP ≤ 2 mg/L [Hazard ratio (HR) 1.69, 95% confidence interval (CI) 1.39–2.05 in the univariable model; HR 1.45, 95% CI 1.19–1.77 in the multivariable model 3). The group with hsCRP > 2 mg/L event at least once during follow-up period had a higher risk of all-cause mortality in univariable model (HR 1.45, 95% CI 1.22–1.72), but the significance was not maintained in multivariable models. At the first follow-up landmark, the group with early elevation of hsCRP had a higher risk of incident CVD than the group without early elevation (HR 1.61, 95% CI 1.28–2.03 in the univariable model; HR 1.37, 95% CI 1.08–1.74 in the multivariable model 3). The significant higher risk for all-cause mortality in the group with early elevation of hsCRP, compared to the group without early elevation, was also shown. The corresponding HR (95% CI) was 1.69 (1.40–2.04) in univariable model and 1.26 (1.04–1.53) in multivariable model 3. Similarly, at the second follow-up landmark, the group with early elevation of hsCRP had a higher risk of incident CVD than the group without early elevation (HR 1.68, 95% CI 1.30–2.17 in the univariable model; HR 1.45, 95% CI 1.12–1.89 in the multivariable model 3). The significant higher risk for all-cause mortality in the group with early elevation of hsCRP, compared to the group without early elevation, was also shown. The corresponding HR (95% CI) was 1.83 (1.51–2.22) in univariable model and 1.34 (1.10–1.63) in multivariable model 3.
Longitudinal association of elevation of hsCRP with CVD mortality, cancer mortality, and mortality from other causes at different time points
In the full dataset, the group with hsCRP levels exceeding 2 mg/L has a CVD mortality rate of 1.24, a cancer mortality rate of 2.76, and a rate of 3.33 for other causes, while the group consistently showing hsCRP levels at or below 2 mg/L demonstrates rates of 1.15 for CVD, 1.78 for cancer, and 1.91 for other causes. At the 1st landmark point, the group with early elevation of hsCRP exhibited a CVD mortality rate of 1.51, a cancer mortality rate of 3.06, and a rate of 3.79 for other causes. In contrast, the group without early elevation showed rates of 1.05 for CVD, 1.99 for cancer, and 1.96 for other causes. At the 2nd landmark point, the group with early elevation of hsCRP registered a CVD mortality rate of 1.42, a cancer mortality rate of 2.85, and a rate of 3.88 for other reasons, while those without the early elevation observed rates of 0.98 for CVD, 1.83 for cancer, and 1.58 for other causes.
Figures 2A–I present the Kaplan–Meier curves with log-rank tests for the cumulative rates of CVD mortality, cancer mortality, and mortality from other causes according to hsCRP elevation events at different time points. There were no significant differences in the cumulative incidence rates of CVD mortality between the two groups in the full dataset and at the landmark analyses at the first and second follow-ups (Fig. 2A–C). The group with hsCRP > 2 mg/L events at least once during follow-up had significantly higher cumulative rates of cancer mortality than the group with consistently hsCRP < 2 mg/L (log-rank test p = 0.002 for full dataset [Figs. 2D]; 0.005 for 1st landmark point [Fig. 2E]; 0.005 for 2nd landmark point [Fig. 2F]). The group with hsCRP > 2 mg/L events at least once during follow-up had significantly higher cumulative rates of mortality from other causes than the group with consistently hsCRP < 2 mg/L (log-rank test p < 0.001 for all datasets; Fig. 2G–I).

Kaplan–Meier curves showing the cumulative incidence rate of CVD mortality, cancer mortality, and mortality from other causes according to the hsCRP elevation event at different time points, including full dataset (A CVD mortality, D cancer mortality, G mortality from other causes), landmark at 1st follow-up (B CVD mortality, E cancer mortality, H mortality from other causes), and landmark at 2nd follow-up (C CVD mortality, E cancer mortality, I mortality from other causes). hsCRP high-sensitivity C-reactive protein, CVD cardiovascular disease.
Table 3 shows the results of the modified Cox regression by Fine and Gray, examining the association between elevated serum hsCRP levels and CVD mortality, cancer mortality, and mortality from other causes at different time points. For the full dataset, the adjusted HR (95% CI) in the group with hsCRP > 2 mg/L events at least once during the follow-up, compared to the group with consistent hsCRP ≤ 2 mg/L, was 0.72 (0.49–1.06) for CVD mortality, 1.19 (0.90–1.58) for cancer mortality, and 1.28 (0.99–1.66) for mortality from other causes, respectively, in multivariable model 3. At the first landmark point, the adjusted HR (95% CI) in the group with early elevation of hsCRP, compared to the group without early elevation, was 0.96 (0.63–1.47) for CVD mortality, 1.18 (0.87–1.61) for cancer mortality, and 1.44 (1.08–1.92) for mortality from other causes, respectively, in multivariable model 3. At the second landmark point, the adjusted HR (95% CI) in the group with early elevation of hsCRP, compared to the group without early elevation, was 0.92 (0.60–1.42) for CVD mortality, 1.17 (0.85–1.61) for cancer mortality, and 1.80 (1.34–2.43) for mortality from other causes, respectively, in multivariable model 3.
Discussion
In this study, early elevation of serum hsCRP levels was associated with an increased risk of incident CVD, independent of traditional risk factors, such as cigarette smoking, DM, HTN, and dyslipidemia. The clinical significance of these findings is noteworthy, as the association remained significant even after considering all-cause mortality as a competing risk. This emphasizes the importance of monitoring longitudinal changes in hsCRP levels for CVD risk assessment. This finding is consistent with previous evidence demonstrating the impact of the early elevation of inflammatory markers on adverse clinical outcomes12,13. Our results also corroborate the well-established association between elevated hsCRP levels and increased risk of CVD8,9,10,11. However, our study extends the current knowledge by demonstrating the importance of longitudinal changes in hsCRP levels in predicting CVD risk.
The JUPITER trials focused on individuals with elevated levels of serum hsCRP (≥ 2 mg/L) but LDL cholesterol levels below 130 mg/dL17. The JUPITER trials demonstrated that early identification and management of individuals with elevated serum hsCRP levels, through statin therapy, can significantly reduce the incidence of CVD. This underscores the importance of routine screening for hsCRP levels in individuals at risk of CVD and the potential benefits of early intervention in this population. In this study, serum LDL cholesterol levels were < 130 mg/dL in individuals with early elevation of serum hsCRP at the first and second landmark points. Our findings support previous ones in the field and emphasize the importance of early identification and management of individuals with elevated serum hsCRP levels.
Although our study found a significant association between early elevated hsCRP levels and the risk of incident CVD and all-cause mortality, no significant association was observed with CVD-specific mortality while significant association was observed with mortality from other causes. One possible explanation for this is the presence of competing risks which were not considered in this study. Individuals with elevated hsCRP levels may have a higher risk of dying from other causes, such as infectious diseases or DM, which may obscure the association with CVD mortality. Lee et al.18 suggested that the association of serum hsCRP with cancer and CVD mortality risk can be attenuated by sex and comorbidities. They analyzed data from a total of 41,070 men and 81,011 women aged 40 years or older, with a follow-up period of 6.8 years, and found that serum hsCRP and risk of all-cause mortality exhibited a strong linear correlation, and that the association between hsCRP and cancer mortality risk was not observed in women with comorbidities, while the relationship with CVD mortality risk was mainly seen in men with comorbidities18. In addition, individuals at high risk for CVD are more likely to receive aggressive medical treatment, such as lipid-lowering therapies, antidiabetic medications, antihypertensive medications, or antithrombotic medications, which can reduce their risks of CVD-related death. The insufficient sample size and follow-up duration in our study might also have contributed to the insignificant association between early elevated hsCRP levels and CVD mortality. Further research with a larger sample size and longer follow-up period may potentially reveal a more significant association.There are several possible explanations for our results. First, it is important to consider that lifestyle factors, such as poor diet, sedentary behavior, and stress, can contribute to the early elevation of hsCRP levels. Prolonged exposure to these factors may exacerbate the inflammatory response and lead to a higher risk of developing CVD. This elevated risk can manifest as increased hsCRP levels, which act as markers of chronic inflammation19. In particular, considering that the only significant proportional difference between the two groups was in terms of current smokers, cigarette smoking might have acted as a major contributor to the elevation of serum hsCRP in this study. Second, genetic factors may also play some roles in the development of early elevation of serum hsCRP level20,21,22,23. Fransén et al.20 found strong associations between CRP and rs3091244, rs1800947, rs1130864, and rs1205 genotypes in women and rs1800947 in men. In addition, Gholami et al.21 also also found a significant relationship between hsCRP and genetic risk score, including single nucleotide polymorphisms in rs17782313, rs3807992, and rs2287161. In this study, certain polymorphisms might have contributed to chronic low-grade inflammation in individuals with early elevation of serum hsCRP levels, resulting in faster attainment of hsCRP levels > 2 mg/L. However, we were unable to confirm the existence of a specific genetic polymorphism because we did not conduct a genome-wide association study (GWAS). To obtain a more comprehensive and robust analysis, further GWAS is necessary.
This study had several key limitations. First, landmark analysis assumed that the onset of hsCRP levels > 2 mg/L would persist in the future. However, this approach might not completely exclude cases with transient hsCRP elevation, which might not have a sustained impact on CVD risk. Second, we did not account for potential changes in medication use during the follow-up period, such as lipid-lowering agents, antihypertensive medications, or antidiabetic medications, which may affect the outcomes of interest. Third, we did not consider the potential influence of other inflammatory markers on the outcomes of interest. Furthermore, we did not analyze temporal trends of changes in serum hsCRP levels or their associations with the development of incident CVD or mortality. Future studies verifying the association between serum hsCRP trajectories and incident CVD and mortality should be performed. Finally, although we utilized expert examiners to verify each case through comprehensive, face-to-face interviews, it’s worth noting the potential for information bias in characterizing incident CVD events due to the reliance on self-reported questionnaires. Nevertheless, our methodology’s credibility is supported by a previous study that reported a 93% match between self-reported diagnoses and those confirmed by physician reviews of medical records24. Nevertheless, we firstly verified early elevation of hsCRP as a risk factor for incident CVD and all-cause mortality. Additionally, to the best of our knowledge, this is the first study to conduct a competing risk analysis of mortality in relation to hsCRP and incident CVD risk.
In conclusion, early elevation of serum hsCRP levels is associated with a higher risk of developing CVD and all-cause mortality. Our findings suggest that the time to hsCRP increase is also important in predicting the occurrence of CVD, even considering competing risk. Therefore, in clinical practice, regular hsCRP testing can help monitor the trend of hsCRP increase and establish personalized treatment plans for preventing CVD in patients. This strategy may help healthcare providers identify individuals at an increased risk of CVD and take necessary steps to prevent the development of CVD and mortality.
Methods
Study population
We conducted an analysis using data from the Korean Genome and Epidemiology Study (KoGES)_Ansan_Ansung cohort, a community-based prospective cohort study conducted by the Korea Disease Control and Prevention Agency25. The cohort was established in 2001–2002, and participants were surveyed biennially until the eighth follow-up in 2017–2018. The study population consisted of community-dwelling adults aged 40–69 years who had lived in the Ansan (urban) and Ansung (rural) areas for at least 6 months.
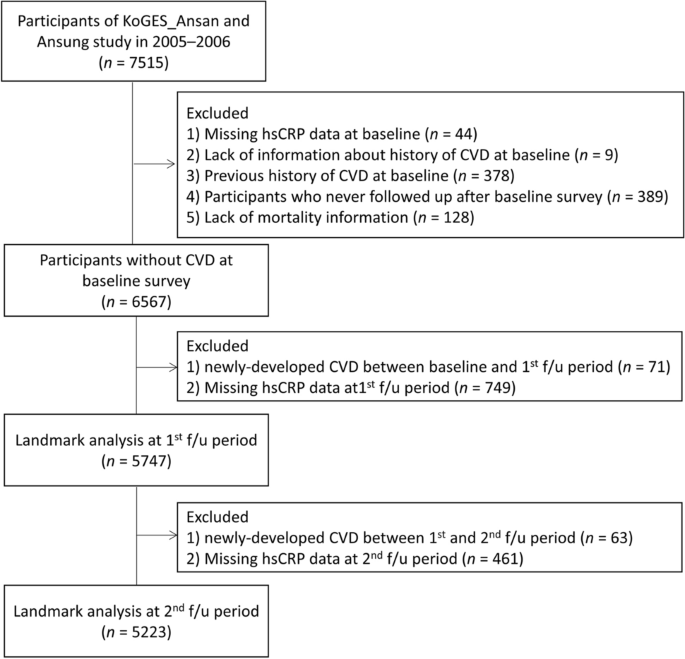
The analysis was limited to data collected between 2005–2006 and 2017–2018 due to the fact that hsCRP was not measured in 2001–2002, and there was a high proportion of missing hsCRP values in 2003–2004 (66%, 5,660 out of 8,603). The baseline data for the analysis were set as the data collected in 2005–2006, as illustrated in Fig. 3. Among the 7515 participants at baselines, we excluded those with missing serum hsCRP data (n = 44), those lacking information regarding CVD history (n = 9), those with CVD history at baseline (n = 378), those who did not follow up during the follow-up period (n = 389), and those with missing mortality information (n = 128). In the end, 6567 participants were included in the analysis.

Flowchart showing the selection of the study population. KoGES Korea Genome and Epidemiology Study, hsCRP high-sensitivity C-reactive protein, CVD cardiovascular disease.
Immortal time bias could lead to underestimation of the rate of clinical outcomes in patients who achieve the surrogate endpoints after the baseline and overestimation in those who fail to achieve these endpoints, which means that the real clinical outcomes may not be accurately reflected due to the skewness caused by immortal time bias26,27. To investigate the effect of early elevation of hsCRP as a risk factor for the development of CVD and account for the potential effect of immortal time bias, we performed landmark analyses28. Furthermore, to ensure sufficient time to accumulate cases of CVD incidence after the landmark points, we established the 1st and 2nd follow-ups as the landmark time points. at the first (2007–2008) and second (2009–2010) follow-up periods subsequent to the establishment of the baseline (2005–2006). The number of participants at the first landmark point was 5747, and the number at the second landmark point was 5223.
The KoGES_Ansan_Ansung cohort protocol was reviewed and approved by the Institutional Review Board (IRB) of the Korea Disease Control and Prevention Agency. All the participants read and signed a written informed consent form. The study protocol conformed to the ethical guidelines of the 1964 Declaration of Helsinki and its later amendments. This study was approved by the IRB of the Nowon Eulji Medical Center (IRB number: 2022-12-010).
Data collection
Personal medical history, dietary habits, smoking status, alcohol consumption, physical activity, monthly household income, and educational level were obtained from each participant using questionnaires. Personal medical history included HTN, DM, dyslipidemia, myocardial infarction, angina pectoris, peripheral artery disease, and ischemic stroke. The age of the patient at the time of diagnosis of each disease was also obtained. The total energy intake (kcal/day) was calculated using a 103-item food frequency questionnaire. Participants were categorized into: never smokers, former smokers, or current smokers; current drinkers or non-drinkers; and regular exercisers or non-exercisers. After at least 8 h of fasting, blood samples were collected from each participant. FPG, serum insulin, total cholesterol, triglyceride, and HDL cholesterol levels were analyzed. In cases of serum triglyceride levels < 400 mg/dL, serum LDL cholesterol was calculated using the Friedewald formula.
Definition of serum hsCRP elevation
Serum hsCRP levels were measured using an immunoradiometric assay (ADVIA 165; Siemens, Erlangen, Germany). We defined a serum hsCRP elevation event as the first occurrence during the follow-up period when hsCRP levels exceeded 2 mg/L, based on the highest concordance index in the preliminary analysis and optimal cut-off points, considering previous patient history as well12,29,30. At baseline, participants were classified into one group that had hsCRP levels > 2 mg/L at least once during follow-up and another group that had consistent hsCRP levels ≤ 2 mg/L. If hsCRP > 2 mg/L was detected at each landmark point, it was defined as an early elevation of hsCRP. The participants were classified into the early elevation hsCRP and non-early elevation hsCRP groups.
Clinical outcomes
The primary outcome of interest was incident CVD, which we defined as newly developed myocardial infarction, angina pectoris, peripheral artery disease, and/or ischemic stroke. When a participant reported an incident CVD event on the personal medical history questionnaire, well-trained examiners conducted in-depth personal interviews to confirm the case. The follow-up time was defined as the time interval from the first follow-up period to the occurrence of a new-onset CVD event. The secondary outcomes were all-cause and CVD mortalities. To ascertain these outcomes, we linked the personal identification key code generated by the KoGES with national data sources, including death records from the Korea National Statistical Office. Information regarding cause-of-death and date-of-death of the participants was tracked from January, 2001 to December, 2020. The underlying causes of death were determined based on the Korean Standard Classification of Diseases codes listed in the National Death Index, and CVD mortality was defined according to the International Classification of Diseases 10th revision (ICD-10) codes I00–I99, while cancer mortality was determined using the ICD-10 codes C00–C97. Mortality from other causes refers to deaths caused by conditions other than CVD and cancer.
Statistical analysis
We analyzed data separately using the full dataset, landmarks at the first follow-up, and landmarks at the second follow-up. Data are presented as mean ± standard deviation for continuous variables and number (%) for categorical variables. Student’s t-tests were used to compare differences in continuous variables, and chi-squared test was used to compare differences in categorical variables between the two groups at baseline and the first and second follow-up periods.
We compared the risk of CVD incidence and mortality between the group with serum hsCRP > 2 mg/L at least once during follow-up and those without. The Kaplan–Meier curves with log-rank tests were used to present the cumulative rates of incident CVD, all-cause mortality, CVD mortality, cancer mortality, and mortality from other causes between the two groups. Given the increasing recognition of the influence of competing risks in the development of prognostic models31, we used modified Cox regression by Fine and Gray to estimate hazard ratios (HRs) with 95% confidence intervals (Cis) for incident CVD, and death was considered as a competing risk. We did not count a mortality case if there was a CVD incidence event before death (48 cases). Furthermore, we also conducted competing risk analysis on all-cause mortality, stratifying it into CVD mortality, cancer mortality, and other-cause mortality. In the multivariable model, we included age, sex, BMI, smoking status, drinking status, physical activity, total energy intake, MBP, FPG, and serum LDL cholesterol level.
All statistical analyses were conducted using R software (version 4.1.1; R Foundation for Statistical Computing, Vienna, Austria). Statistical significance was set at p < 0.05.
Ethcal approval
The KoGES_Ansan_Ansung cohort protocol was reviewed and approved by the Institutional Review Board (IRB) of the Korea Disease Control and Prevention Agency. All the participants read and signed a written informed consent form. The study protocol conformed to the ethical guidelines of the 1964 Declaration of Helsinki and its later amendments. This study was approved by the IRB of the Nowon Eulji Medical Center (IRB number: 2022-12-010).
Data availability
The dataset used in this study are available from the Korea Disease Control and Prevention Agency with permission and can be accessed at https://www.kdca.go.kr/contents.es?mid=a40504020100.
Abbreviations
- CVD:
-
Cardiovascular disease
- hsCRP:
-
High-sensitivity C-reactive protein
- IL-6:
-
Interleukin-6
- TNF-α:
-
Tumor necrosis factor-alpha
- HTN:
-
Hypertension
- DM:
-
Diabetes mellitus
- BMI:
-
Body mass index
- WC:
-
Waist circumference
- MBP:
-
Mean blood pressure
- FPG:
-
Fasting plasma glucose
- HDL:
-
High-density lipoprotein
- LDL:
-
Low-density lipoprotein
- HR:
-
Hazard ratio
- CI:
-
Confidence interval
- GWAS:
-
Genome-wide association study
- KoGES:
-
Korean Genome and Epidemiology Study
- IRB:
-
Institutional Review Board
References
-
Roth, G. A. et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the Global Burden of Disease Study 2017. The Lancet 392, 1736–1788. https://doi.org/10.1016/S0140-6736(18)32203-7 (2018).
Google Scholar
-
Roth, G. A. et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: Update from the GBD 2019 study. J. Am. Coll. Cardiol. 76, 2982–3021. https://doi.org/10.1016/j.jacc.2020.11.010 (2020).
Google Scholar
-
Li, Y., Cao, G. Y., Jing, W. Z., Liu, J. & Liu, M. Global trends and regional differences in incidence and mortality of cardiovascular disease, 1990–2019: Findings from 2019 global burden of disease study. Eur. J. Prev. Cardiol. 30, 276–286. https://doi.org/10.1093/eurjpc/zwac285 (2023).
Google Scholar
-
Fan, J. & Watanabe, T. Atherosclerosis: Known and unknown. Pathol. Int. 72, 151–160. https://doi.org/10.1111/pin.13202 (2022).
Google Scholar
-
Moriya, J. Critical roles of inflammation in atherosclerosis. J. Cardiol. 73, 22–27. https://doi.org/10.1016/j.jjcc.2018.05.010 (2019).
Google Scholar
-
Mahalle, N., Garg, M., Kulkarni, M. & Naik, S. Association of inflammatory cytokines with traditional and nontraditional cardiovascular risk factors in Indians with known coronary artery disease. Ann. Med. Health Sci. Res. 4, 706–712. https://doi.org/10.4103/2141-9248.141525 (2014).
Google Scholar
-
Fuchs, F. D. & Whelton, P. K. High blood pressure and cardiovascular disease. Hypertension 75, 285–292. https://doi.org/10.1161/hypertensionaha.119.14240 (2020).
Google Scholar
-
de Goma, E. M., Knowles, J. W., Angeli, F., Budoff, M. J. & Rader, D. J. The evolution and refinement of traditional risk factors for cardiovascular disease. Cardiol. Rev. 20, 118–129. https://doi.org/10.1097/CRD.0b013e318239b924 (2012).
Google Scholar
-
Romero-Cabrera, J. L., Ankeny, J., Fernández-Montero, A., Kales, S. N. & Smith, D. L. A systematic review and meta-analysis of advanced biomarkers for predicting incident cardiovascular disease among asymptomatic middle-aged adults. Int. J. Mol. Sci. 23, 13450. https://doi.org/10.3390/ijms232113540 (2022).
Google Scholar
-
Musunuru, K. et al. The use of high-sensitivity assays for C-reactive protein in clinical practice. Nat. Clin. Pract Cardiovasc Med 5, 621–635. https://doi.org/10.1038/ncpcardio1322 (2008).
Google Scholar
-
Yousuf, O. et al. High-sensitivity C-reactive protein and cardiovascular disease: A resolute belief or an elusive link?. J. Am. Coll. Cardiol. 62, 397–408. https://doi.org/10.1016/j.jacc.2013.05.016 (2013).
Google Scholar
-
Zhang, W. et al. High-sensitivity C-reactive protein modifies the cardiovascular risk of lipoprotein(a): Multi-ethnic study of atherosclerosis. J. Am. Coll. Cardiol. 78, 1083–1094. https://doi.org/10.1016/j.jacc.2021.07.016 (2021).
Google Scholar
-
Silva, D. & Pais de Lacerda, A. High-sensitivity C-reactive protein as a biomarker of risk in coronary artery disease. Rev. Port. Cardiol. 31, 733–745. https://doi.org/10.1016/j.repc.2012.02.018 (2012).
Google Scholar
-
Wang, A. et al. Cumulative exposure to high-sensitivity C-reactive protein predicts the risk of cardiovascular disease. J. Am. Heart Assoc. 6, 5610. https://doi.org/10.1161/jaha.117.005610 (2017).
Google Scholar
-
Emsley, H. C. et al. An early and sustained peripheral inflammatory response in acute ischaemic stroke: Relationships with infection and atherosclerosis. J. Neuroimmunol. 139, 93–101. https://doi.org/10.1016/s0165-5728(03)00134-6 (2003).
Google Scholar
-
Cuschieri, J. et al. Early elevation in random plasma IL-6 after severe injury is associated with development of organ failure. Shock 34, 346–351. https://doi.org/10.1097/SHK.0b013e3181d8e687 (2010).
Google Scholar
-
Ridker, P. M., Pradhan, A., MacFadyen, J. G., Libby, P. & Glynn, R. J. Cardiovascular benefits and diabetes risks of statin therapy in primary prevention: An analysis from the JUPITER trial. Lancet 380, 565–571. https://doi.org/10.1016/s0140-6736(12)61190-8 (2012).
Google Scholar
-
Lee, S. A. et al. Association of serum high-sensitivity C reactive protein with risk of mortality in an Asian population: The Health Examinees cohort. BMJ Open 12, e052630. https://doi.org/10.1136/bmjopen-2021-052630 (2022).
Google Scholar
-
Lechner, K. et al. Lifestyle factors and high-risk atherosclerosis: Pathways and mechanisms beyond traditional risk factors. Eur. J. Prev. Cardiol. 27, 394–406. https://doi.org/10.1177/2047487319869400 (2020).
Google Scholar
-
Fransén, K., Pettersson, C. & Hurtig-Wennlöf, A. CRP levels are significantly associated with CRP genotype and estrogen use in The Lifestyle, Biomarker and Atherosclerosis (LBA) study. BMC Cardiovasc Disord 22, 170. https://doi.org/10.1186/s12872-022-02610-z (2022).
Google Scholar
-
Gholami, F. et al. The relationship of genetic risk score with cardiometabolic risk factors: A cross-sectional study. BMC Cardiovasc. Disord. 22, 459. https://doi.org/10.1186/s12872-022-02888-z (2022).
Google Scholar
-
de Santis, I. P. et al. C-reactive protein gene rs1205 polymorphism is associated with low-grade chronic inflammation in postmenopausal women. Womens Midlife Health 6, 3. https://doi.org/10.1186/s40695-020-00051-2 (2020).
Google Scholar
-
Liu, R. et al. A Polymorphism in hepatocyte nuclear factor 1 alpha, rs7310409, is associated with left main coronary artery disease. Biochem. Res. Int. 2014, 924105. https://doi.org/10.1155/2014/924105 (2014).
Google Scholar
-
Baik, I., Cho, N. H., Kim, S. H. & Shin, C. Dietary information improves cardiovascular disease risk prediction models. Eur. J. Clin. Nutr. 67, 25–30. https://doi.org/10.1038/ejcn.2012.175 (2013).
Google Scholar
-
Kim, Y. & Han, B. G. Cohort Profile: The Korean Genome and Epidemiology Study (KoGES) Consortium. Int. J. Epidemiol. 46, e20. https://doi.org/10.1093/ije/dyv316 (2017).
Google Scholar
-
Suissa, S. & Azoulay, L. Response to Yang and Chan. Metformin and the risk of cancer: Time-related biases in observational studies Diabetes care 2012;35:2665-2673. Diabetes Care 36, e88. https://doi.org/10.2337/dc13-0133 (2013).
Google Scholar
-
Giobbie-Hurder, A., Gelber, R. D. & Regan, M. M. Challenges of guarantee-time bias. J. Clin. Oncol. 31, 2963–2969. https://doi.org/10.1200/jco.2013.49.5283 (2013).
Google Scholar
-
Mi, X., Hammill, B. G., Curtis, L. H., Lai, E. C. & Setoguchi, S. Use of the landmark method to address immortal person-time bias in comparative effectiveness research: A simulation study. Stat. Med. 35, 4824–4836. https://doi.org/10.1002/sim.7019 (2016).
Google Scholar
-
Carrero, J. J., Franko, M. A., Obergfell, A., Gabrielsen, A. & Jernberg, T. hsCRP level and the risk of death or recurrent cardiovascular events in patients with myocardial infarction: A healthcare-based study. J. Am. Heart Assoc. 8, e012638. https://doi.org/10.1161/JAHA.119.012638 (2019).
Google Scholar
-
Ridker, P. M., Koenig, W., Kastelein, J. J., Mach, F. & Lüscher, T. F. Has the time finally come to measure hsCRP universally in primary and secondary cardiovascular prevention?. Eur. Heart J. 39, 4109–4111. https://doi.org/10.1093/eurheartj/ehy723 (2018).
Google Scholar
-
Austin, P. C., Lee, D. S. & Fine, J. P. Introduction to the analysis of survival data in the presence of competing risks. Circulation 133, 601–609. https://doi.org/10.1161/circulationaha.115.017719 (2016).
Google Scholar
Funding
This research was supported by EMBRI Grants (2023EMBRISN0002) from the Eulji University.
Author information
Authors and Affiliations
Contributions
H.S.L.: study concept and design; acquisition, analysis, and interpretation of data; drafting the manuscript; J.H.L.: study concept and design; interpretation of data; supervision; revising the manuscript; All authors approved he final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
Reprints and Permissions
About this article
Cite this article
Lee, H.S., Lee, JH. Early elevation of high-sensitivity C-reactive protein as a predictor for cardiovascular disease incidence and all-cause mortality: a landmark analysis.
Sci Rep 13, 14118 (2023). https://doi.org/10.1038/s41598-023-41081-w
-
Received: 22 April 2023
-
Accepted: 21 August 2023
-
Published: 29 August 2023
-
DOI: https://doi.org/10.1038/s41598-023-41081-w
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.