Blood
US to name first 10 drugs for Medicare price negotiation
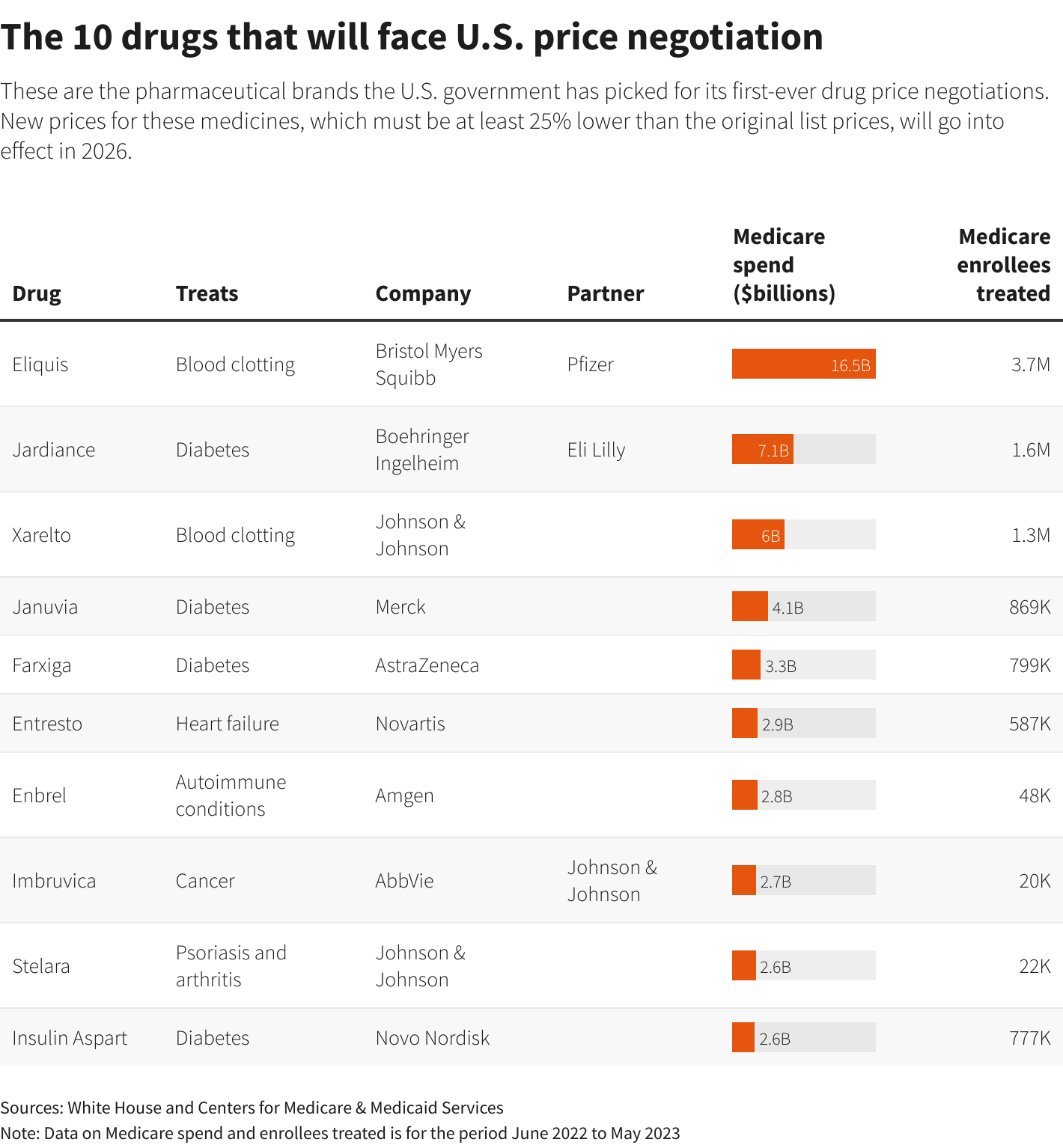
Aug 29(Reuters) – The Biden administration on Tuesday released its list of 10 prescription medicines that will be subject to the first-ever price negotiations by the U.S. Medicare health program that covers 66 million people, with big-selling blood thinner Eliquis from Bristol Myers Squibb (BMY.N) and Pfizer (PFE.N) among them.
President Joe Biden’s signature Inflation Reduction Act (IRA), signed into law last year, allows Medicare to negotiate prices for some of its most costly drugs.
“There is no reason why Americans should be forced to pay more than any developed nation for life-saving prescriptions just to pad Big Pharma’s pockets,” Biden said in a statement.
He said that once implemented, the prices on negotiated drugs will decrease for up to 9 million seniors who currently pay as much as $6,497 in out-of-pocket costs per year for these prescriptions. Medicare mostly serves Americans aged 65 and over.
Merck & Co’s (MRK.N) diabetes drug Januvia, Eliquis rival Xarelto from Johnson & Johnson (JNJ.N), and AbbVie’s (ABBV.N) leukemia treatment Imbruvica were also selected.
Other medicines picked for negotiations include Amgen’s (AMGN.O) rheumatoid arthritis treatment Enbrel, Boehringer Ingelheim and Eli Lilly’s (LLY.N) diabetes drug Jardiance, J&J’s arthritis and Crohn’s disease medicine Stelara and insulin from Novo Nordisk (NOVOb.CO).
Shares of drugmakers were mostly flat in morning trading. The NYSE Arca Pharmaceutical index (.DRG) was down 0.1%.
This kicks off the negotiation process for the 10 drugs whose new prices will go into effect in 2026. The program aims to save $25 billion per year on drug prices by 2031.
U.S. laws had prohibited Medicare from negotiating pharmaceutical prices as part of its prescription drug program that began about 20 years ago.
The U.S. Centers for Medicare & Medicaid Services (CMS) spent $50.5 billion between June 1, 2022 and May 31, 2023 on the 10 drugs, which is the time period used to determine which medicines were eligible for negotiation. That was about 20% of the total cost of drugs in the Medicare prescription drug program known as Part D.
Wells Fargo analyst Mohit Bansal said the savings from negotiations on Jardiance, Januvia, AstraZeneca’s (AZN.L) Farxiga and Novo’s Insulin Aspart, which cost the agency about $16.5 billion total, could potentially free up Medicare’s budget and make it easier to cover diabetes or obesity drugs.
CLINICALLY APPROPRIATE ACCESS
Novartis , whose heart failure drug Entresto was among the 10 selected, Eli Lilly and Merck said they believed the price-setting would stifle innovation in the sector and impact quality of care. AstraZeneca and Novo Nordisk said they were evaluating next steps.
Bristol Myers CEO Giovanni Caforio in an interview said the inclusion of Eliquis would not impact its long-term strategy, particularly as the drug loses patent exclusivity in 2028, two years after the negotiated prices would take effect.
Caforio said Medicare enrollees on the drug could see their access restricted because of unintended consequences of the law.
“There is no requirement in the law that insurance companies that administer Medicare benefits will actually continue to make these medicines available to patients without hurdles or burdensome cost sharing,” he said.
CMS Director Dr. Meena Seshamani said Medicare plans to use a review process to make sure insurance companies keep clinically appropriate access to negotiated drugs.
Competition for J&J’s Stelara is expected to hit the U.S. market in 2025, before negotiated prices go into effect, following deals with Amgen, Alvotech (ALVO.O) and Teva (TEVA.TA) that delayed launches of their near copies, known as biosimilars.
Analysts had said the delays put J&J back on track for $57 billion in 2025 pharmaceutical revenue.
Bristol Myers, J&J, Merck, AstraZeneca, and Germany-based Boehringer have also sued the U.S. Department of Health and Human Services (HHS), which oversees the Medicare agency, in an effort to derail the price-setting process.
BMO Capital Markets analyst Evan Seigerman said that while the list includes many big revenue generators, many of them will face competition shortly after or even before 2026, which was expected to lessen their profitability.
Two analysts said they expect the negotiated prices to move beyond Medicare and affect commercial markets for these drugs by 2026, when they come into effect.
The 10 initial drugs were chosen based on certain criteria set out by Medicare. They must be sold in pharmacies, not have substantial generic competition, and have been on the market for at least nine years – 13 for more complex biotech drugs.
Reporting by Patrick Wingrove, Mike Erman, Manas Mishra in Bengaluru and Nandita Bose in Washington;
Editing by Caroline Humer, Bill Berkrot and Chizu Nomiyama
Our Standards: The Thomson Reuters Trust Principles.