Blood
LivaNova Nabs FDA Nod, CE-Mark for In-Line Blood Monitor
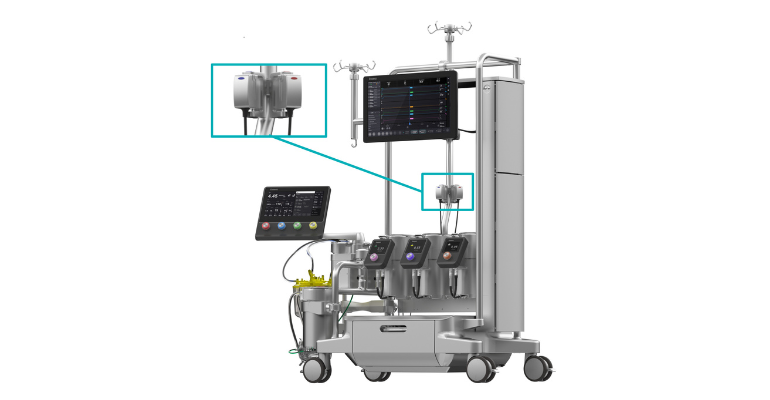
LivaNova recently received FDA 510(k) clearance and CE-mark for its Essenz In-Line Blood Monitor (ILBM). The monitor is integrated into the company’s next-generation cardiopulmonary bypass procedure (CPB) platform, the Essenz Perfusion System and provides accurate and continuous measurement of essential blood parameters from its cockpit to medical professionals during CPB.
Along with the new inclusion of ILBM, the perfusion system also has a next-generation heart-lung machine, a patient monitor, and accurate sensing technology.
Blood gas analyzers currently used similarly only reflect a patient’s condition at the moment of blood draw, which can quickly change and become irrelevant, according to LivaNova. The Essenz monitor relays continuous monitoring information for the duration of a procedure, allowing for the delivery of patient-tailored intervention based on up-to-date data.
“Dynamic conditions can rapidly change a patient’s blood parameters during a cardiopulmonary bypass procedure,” said Marco Dolci, LivaNova president, Cardiopulmonary, in a press release. “Essenz… provides continuous monitoring throughout a patient’s procedure. Access to accurate, real-time measurements directly from the Essenz Perfusion System allows for quick decisions and tailored care strategies to serve the patient.”
The system uses B-Capta sensing technology and works within Clinical Laboratory Improvement Amendments guidelines while providing measured values on par with existing hospital blood gas analyzers. It provides measured values for oxygen saturation, hematocrit, partial pressure of oxygen, and temperature.
Additionally, ILBM does not have to be calibrated to set device measurements, allowing for quicker device set up. “Arterial and venous parameters are automatically transferred to the Essenz Patient Monitor, supporting data-driven decision making and the implementation of goal-directed perfusion (GDP), a therapy effective in reducing the risk of acute kidney injury,” according to the press release. “The latest heart-lung machine software, version 1.3, integrates the ILBM with the Essenz Perfusion System and was developed to continually enhance the user experience.”
With the FDA clearance and CE-mark, the Essenz Perfusion System is currently available in Europe, the United States, Canada, Australia, Japan, and the United Arab Emirates. The device was initially launched in February 2023.