Congenital disorders
AAV-based in vivo gene therapy for neurological disorders
Abstract
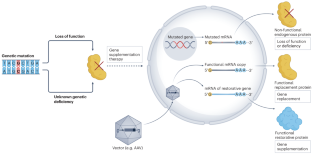
Recent advancements in gene supplementation therapy are expanding the options for the treatment of neurological disorders. Among the available delivery vehicles, adeno-associated virus (AAV) is often the favoured vector. However, the results have been variable, with some trials dramatically altering the course of disease whereas others have shown negligible efficacy or even unforeseen toxicity. Unlike traditional drug development with small molecules, therapeutic profiles of AAV gene therapies are dependent on both the AAV capsid and the therapeutic transgene. In this rapidly evolving field, numerous clinical trials of gene supplementation for neurological disorders are ongoing. Knowledge is growing about factors that impact the translation of preclinical studies to humans, including the administration route, timing of treatment, immune responses and limitations of available model systems. The field is also developing potential solutions to mitigate adverse effects, including AAV capsid engineering and designs to regulate transgene expression. At the same time, preclinical research is addressing new frontiers of gene supplementation for neurological disorders, with a focus on mitochondrial and neurodevelopmental disorders. In this Review, we describe the current state of AAV-mediated neurological gene supplementation therapy, including critical factors for optimizing the safety and efficacy of treatments, as well as unmet needs in this field.
This is a preview of subscription content, access via your institution
Access options
style{display:none!important}.LiveAreaSection-193358632 *{align-content:stretch;align-items:stretch;align-self:auto;animation-delay:0s;animation-direction:normal;animation-duration:0s;animation-fill-mode:none;animation-iteration-count:1;animation-name:none;animation-play-state:running;animation-timing-function:ease;azimuth:center;backface-visibility:visible;background-attachment:scroll;background-blend-mode:normal;background-clip:borderBox;background-color:transparent;background-image:none;background-origin:paddingBox;background-position:0 0;background-repeat:repeat;background-size:auto auto;block-size:auto;border-block-end-color:currentcolor;border-block-end-style:none;border-block-end-width:medium;border-block-start-color:currentcolor;border-block-start-style:none;border-block-start-width:medium;border-bottom-color:currentcolor;border-bottom-left-radius:0;border-bottom-right-radius:0;border-bottom-style:none;border-bottom-width:medium;border-collapse:separate;border-image-outset:0s;border-image-repeat:stretch;border-image-slice:100%;border-image-source:none;border-image-width:1;border-inline-end-color:currentcolor;border-inline-end-style:none;border-inline-end-width:medium;border-inline-start-color:currentcolor;border-inline-start-style:none;border-inline-start-width:medium;border-left-color:currentcolor;border-left-style:none;border-left-width:medium;border-right-color:currentcolor;border-right-style:none;border-right-width:medium;border-spacing:0;border-top-color:currentcolor;border-top-left-radius:0;border-top-right-radius:0;border-top-style:none;border-top-width:medium;bottom:auto;box-decoration-break:slice;box-shadow:none;box-sizing:border-box;break-after:auto;break-before:auto;break-inside:auto;caption-side:top;caret-color:auto;clear:none;clip:auto;clip-path:none;color:initial;column-count:auto;column-fill:balance;column-gap:normal;column-rule-color:currentcolor;column-rule-style:none;column-rule-width:medium;column-span:none;column-width:auto;content:normal;counter-increment:none;counter-reset:none;cursor:auto;display:inline;empty-cells:show;filter:none;flex-basis:auto;flex-direction:row;flex-grow:0;flex-shrink:1;flex-wrap:nowrap;float:none;font-family:initial;font-feature-settings:normal;font-kerning:auto;font-language-override:normal;font-size:medium;font-size-adjust:none;font-stretch:normal;font-style:normal;font-synthesis:weight style;font-variant:normal;font-variant-alternates:normal;font-variant-caps:normal;font-variant-east-asian:normal;font-variant-ligatures:normal;font-variant-numeric:normal;font-variant-position:normal;font-weight:400;grid-auto-columns:auto;grid-auto-flow:row;grid-auto-rows:auto;grid-column-end:auto;grid-column-gap:0;grid-column-start:auto;grid-row-end:auto;grid-row-gap:0;grid-row-start:auto;grid-template-areas:none;grid-template-columns:none;grid-template-rows:none;height:auto;hyphens:manual;image-orientation:0deg;image-rendering:auto;image-resolution:1dppx;ime-mode:auto;inline-size:auto;isolation:auto;justify-content:flexStart;left:auto;letter-spacing:normal;line-break:auto;line-height:normal;list-style-image:none;list-style-position:outside;list-style-type:disc;margin-block-end:0;margin-block-start:0;margin-bottom:0;margin-inline-end:0;margin-inline-start:0;margin-left:0;margin-right:0;margin-top:0;mask-clip:borderBox;mask-composite:add;mask-image:none;mask-mode:matchSource;mask-origin:borderBox;mask-position:0 0;mask-repeat:repeat;mask-size:auto;mask-type:luminance;max-height:none;max-width:none;min-block-size:0;min-height:0;min-inline-size:0;min-width:0;mix-blend-mode:normal;object-fit:fill;object-position:50% 50%;offset-block-end:auto;offset-block-start:auto;offset-inline-end:auto;offset-inline-start:auto;opacity:1;order:0;orphans:2;outline-color:initial;outline-offset:0;outline-style:none;outline-width:medium;overflow:visible;overflow-wrap:normal;overflow-x:visible;overflow-y:visible;padding-block-end:0;padding-block-start:0;padding-bottom:0;padding-inline-end:0;padding-inline-start:0;padding-left:0;padding-right:0;padding-top:0;page-break-after:auto;page-break-before:auto;page-break-inside:auto;perspective:none;perspective-origin:50% 50%;pointer-events:auto;position:static;quotes:initial;resize:none;right:auto;ruby-align:spaceAround;ruby-merge:separate;ruby-position:over;scroll-behavior:auto;scroll-snap-coordinate:none;scroll-snap-destination:0 0;scroll-snap-points-x:none;scroll-snap-points-y:none;scroll-snap-type:none;shape-image-threshold:0;shape-margin:0;shape-outside:none;tab-size:8;table-layout:auto;text-align:initial;text-align-last:auto;text-combine-upright:none;text-decoration-color:currentcolor;text-decoration-line:none;text-decoration-style:solid;text-emphasis-color:currentcolor;text-emphasis-position:over right;text-emphasis-style:none;text-indent:0;text-justify:auto;text-orientation:mixed;text-overflow:clip;text-rendering:auto;text-shadow:none;text-transform:none;text-underline-position:auto;top:auto;touch-action:auto;transform:none;transform-box:borderBox;transform-origin:50% 50%0;transform-style:flat;transition-delay:0s;transition-duration:0s;transition-property:all;transition-timing-function:ease;vertical-align:baseline;visibility:visible;white-space:normal;widows:2;width:auto;will-change:auto;word-break:normal;word-spacing:normal;word-wrap:normal;writing-mode:horizontalTb;z-index:auto;-webkit-appearance:none;-moz-appearance:none;-ms-appearance:none;appearance:none;margin:0}.LiveAreaSection-193358632{width:100%}.LiveAreaSection-193358632 .login-option-buybox{display:block;width:100%;font-size:17px;line-height:30px;color:#222;padding-top:30px;font-family:Harding,Palatino,serif}.LiveAreaSection-193358632 .additional-access-options{display:block;font-weight:700;font-size:17px;line-height:30px;color:#222;font-family:Harding,Palatino,serif}.LiveAreaSection-193358632 .additional-login>li:not(:first-child)::before{transform:translateY(-50%);content:””;height:1rem;position:absolute;top:50%;left:0;border-left:2px solid #999}.LiveAreaSection-193358632 .additional-login>li:not(:first-child){padding-left:10px}.LiveAreaSection-193358632 .additional-login>li{display:inline-block;position:relative;vertical-align:middle;padding-right:10px}.BuyBoxSection-683559780{display:flex;flex-wrap:wrap;flex:1;flex-direction:row-reverse;margin:-30px -15px 0}.BuyBoxSection-683559780 .box-inner{width:100%;height:100%}.BuyBoxSection-683559780 .readcube-buybox{background-color:#f3f3f3;flex-shrink:1;flex-grow:1;flex-basis:255px;background-clip:content-box;padding:0 15px;margin-top:30px}.BuyBoxSection-683559780 .subscribe-buybox{background-color:#f3f3f3;flex-shrink:1;flex-grow:4;flex-basis:300px;background-clip:content-box;padding:0 15px;margin-top:30px}.BuyBoxSection-683559780 .subscribe-buybox-nature-plus{background-color:#f3f3f3;flex-shrink:1;flex-grow:4;flex-basis:100%;background-clip:content-box;padding:0 15px;margin-top:30px}.BuyBoxSection-683559780 .title-readcube,.BuyBoxSection-683559780 .title-buybox{display:block;margin:0;margin-right:10%;margin-left:10%;font-size:24px;line-height:32px;color:#222;padding-top:30px;text-align:center;font-family:Harding,Palatino,serif}.BuyBoxSection-683559780 .title-asia-buybox{display:block;margin:0;margin-right:5%;margin-left:5%;font-size:24px;line-height:32px;color:#222;padding-top:30px;text-align:center;font-family:Harding,Palatino,serif}.BuyBoxSection-683559780 .asia-link{color:#069;cursor:pointer;text-decoration:none;font-size:1.05em;font-family:-apple-system,BlinkMacSystemFont,”Segoe UI”,Roboto,Oxygen-Sans,Ubuntu,Cantarell,”Helvetica Neue”,sans-serif;line-height:1.05em6}.BuyBoxSection-683559780 .access-readcube{display:block;margin:0;margin-right:10%;margin-left:10%;font-size:14px;color:#222;padding-top:10px;text-align:center;font-family:-apple-system,BlinkMacSystemFont,”Segoe UI”,Roboto,Oxygen-Sans,Ubuntu,Cantarell,”Helvetica Neue”,sans-serif;line-height:20px}.BuyBoxSection-683559780 .access-asia-buybox{display:block;margin:0;margin-right:5%;margin-left:5%;font-size:14px;color:#222;padding-top:10px;text-align:center;font-family:-apple-system,BlinkMacSystemFont,”Segoe UI”,Roboto,Oxygen-Sans,Ubuntu,Cantarell,”Helvetica Neue”,sans-serif;line-height:20px}.BuyBoxSection-683559780 .access-buybox{display:block;margin:0;margin-right:10%;margin-left:10%;font-size:14px;color:#222;opacity:.8px;padding-top:10px;text-align:center;font-family:-apple-system,BlinkMacSystemFont,”Segoe UI”,Roboto,Oxygen-Sans,Ubuntu,Cantarell,”Helvetica Neue”,sans-serif;line-height:20px}.BuyBoxSection-683559780 .price-buybox{display:block;font-size:30px;color:#222;font-family:-apple-system,BlinkMacSystemFont,”Segoe UI”,Roboto,Oxygen-Sans,Ubuntu,Cantarell,”Helvetica Neue”,sans-serif;padding-top:30px;text-align:center}.BuyBoxSection-683559780 .price-buybox-to{display:block;font-size:30px;color:#222;font-family:-apple-system,BlinkMacSystemFont,”Segoe UI”,Roboto,Oxygen-Sans,Ubuntu,Cantarell,”Helvetica Neue”,sans-serif;text-align:center}.BuyBoxSection-683559780 .price-info-text{font-size:16px;padding-right:10px;color:#222;font-family:-apple-system,BlinkMacSystemFont,”Segoe UI”,Roboto,Oxygen-Sans,Ubuntu,Cantarell,”Helvetica Neue”,sans-serif}.BuyBoxSection-683559780 .price-value{font-size:30px;font-family:-apple-system,BlinkMacSystemFont,”Segoe UI”,Roboto,Oxygen-Sans,Ubuntu,Cantarell,”Helvetica Neue”,sans-serif}.BuyBoxSection-683559780 .price-per-period{font-family:-apple-system,BlinkMacSystemFont,”Segoe UI”,Roboto,Oxygen-Sans,Ubuntu,Cantarell,”Helvetica Neue”,sans-serif}.BuyBoxSection-683559780 .price-from{font-size:14px;padding-right:10px;color:#222;font-family:-apple-system,BlinkMacSystemFont,”Segoe UI”,Roboto,Oxygen-Sans,Ubuntu,Cantarell,”Helvetica Neue”,sans-serif;line-height:20px}.BuyBoxSection-683559780 .issue-buybox{display:block;font-size:13px;text-align:center;color:#222;font-family:-apple-system,BlinkMacSystemFont,”Segoe UI”,Roboto,Oxygen-Sans,Ubuntu,Cantarell,”Helvetica Neue”,sans-serif;line-height:19px}.BuyBoxSection-683559780 .no-price-buybox{display:block;font-size:13px;line-height:18px;text-align:center;padding-right:10%;padding-left:10%;padding-bottom:20px;padding-top:30px;color:#222;font-family:-apple-system,BlinkMacSystemFont,”Segoe UI”,Roboto,Oxygen-Sans,Ubuntu,Cantarell,”Helvetica Neue”,sans-serif}.BuyBoxSection-683559780 .vat-buybox{display:block;margin-top:5px;margin-right:20%;margin-left:20%;font-size:11px;color:#222;padding-top:10px;padding-bottom:15px;text-align:center;font-family:-apple-system,BlinkMacSystemFont,”Segoe UI”,Roboto,Oxygen-Sans,Ubuntu,Cantarell,”Helvetica Neue”,sans-serif;line-height:17px}.BuyBoxSection-683559780 .tax-buybox{display:block;width:100%;color:#222;padding:20px 16px;text-align:center;font-family:-apple-system,BlinkMacSystemFont,”Segoe UI”,Roboto,Oxygen-Sans,Ubuntu,Cantarell,”Helvetica Neue”,sans-serif;line-height:NaNpx}.BuyBoxSection-683559780 .button-container{display:flex;padding-right:20px;padding-left:20px;justify-content:center}.BuyBoxSection-683559780 .button-container>*{flex:1px}.BuyBoxSection-683559780 .button-container>a:hover,.Button-505204839:hover,.Button-1078489254:hover,.Button-2496381730:hover{text-decoration:none}.BuyBoxSection-683559780 .readcube-button{background:#fff;margin-top:30px}.BuyBoxSection-683559780 .button-asia{background:#069;border:1px solid #069;border-radius:0;cursor:pointer;display:block;padding:9px;outline:0;text-align:center;text-decoration:none;min-width:80px;margin-top:75px}.BuyBoxSection-683559780 .button-label-asia,.ButtonLabel-3869432492,.ButtonLabel-3296148077,.ButtonLabel-1651148777{display:block;color:#fff;font-size:17px;line-height:20px;font-family:-apple-system,BlinkMacSystemFont,”Segoe UI”,Roboto,Oxygen-Sans,Ubuntu,Cantarell,”Helvetica Neue”,sans-serif;text-align:center;text-decoration:none;cursor:pointer}.Button-505204839,.Button-1078489254,.Button-2496381730{background:#069;border:1px solid #069;border-radius:0;cursor:pointer;display:block;padding:9px;outline:0;text-align:center;text-decoration:none;min-width:80px;max-width:320px;margin-top:10px}.Button-505204839 .readcube-label,.Button-1078489254 .readcube-label,.Button-2496381730 .readcube-label{color:#069}
/* style specs end */
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Rent or buy this article
Prices vary by article type
from$1.95
to$39.95
Prices may be subject to local taxes which are calculated during checkout



References
-
Janson, C. et al. Gene therapy of Canavan disease: AAV-2 vector for neurosurgical delivery of aspartoacylase gene (ASPA) to the human brain. Hum. Gene Ther. 13, 1391–1412 (2002).
Google Scholar
-
Leone, P. et al. Long-term follow-up after gene therapy for Canavan disease. Sci. Transl. Med. 4, 165ra163 (2012).
Google Scholar
-
Cavazzana, M., Bushman, F. D., Miccio, A., André-Schmutz, I. & Six, E. Gene therapy targeting haematopoietic stem cells for inherited diseases: progress and challenges. Nat. Rev. Drug. Discov. 18, 447–462 (2019).
Google Scholar
-
Kinsella, J. L. et al. Ex-vivo autologous stem cell gene therapy clinical trial for mucopolysaccharidosis type IIIA: trial in progress-NCT04201405. Blood 136, 15–16 (2020).
Google Scholar
-
Gentner, B. et al. Hematopoietic stem- and progenitor-cell gene therapy for Hurler syndrome. N. Engl. J. Med. 385, 1929–1940 (2021).
Google Scholar
-
Fumagalli, F. et al. Lentiviral haematopoietic stem-cell gene therapy for early-onset metachromatic leukodystrophy: long-term results from a non-randomised, open-label, phase 1/2 trial and expanded access. Lancet 399, 372–383 (2022).
Google Scholar
-
Eichler, F. et al. Hematopoietic stem-cell gene therapy for cerebral adrenoleukodystrophy. N. Engl. J. Med. 377, 1630–1638 (2017).
Google Scholar
-
Charlesworth, C. T., Hsu, I., Wilkinson, A. C. & Nakauchi, H. Immunological barriers to haematopoietic stem cell gene therapy. Nat. Rev. Immunol. 12, 719–733 (2022).
Google Scholar
-
Bulcha, J. T., Wang, Y., Ma, H., Tai, P. W. & Gao, G. Viral vector platforms within the gene therapy landscape. Signal. Transduct. Target. Ther. 6, 1–24 (2021).
Google Scholar
-
Sung, Y. K. & Kim, S. Recent advances in the development of gene delivery systems. Biomater. Res. 23, 1–7 (2019).
Google Scholar
-
Atchison, R. W., Casto, B. C. & Hammon, W. M. Adenovirus-associated defective virus particles. Science 149, 754–756 (1965).
Google Scholar
-
Flotte, T. R. Gene therapy progress and prospects: recombinant adeno-associated virus (rAAV) vectors. Gene Ther. 11, 805–810 (2004).
Google Scholar
-
Duque, S. et al. Intravenous administration of self-complementary AAV9 enables transgene delivery to adult motor neurons. Mol. Ther. 17, 1187–1196 (2009).
Google Scholar
-
Foust, K. D. et al. Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nat. Biotechnol. 27, 59–65 (2009).
Google Scholar
-
Gao, G. et al. Clades of adeno-associated viruses are widely disseminated in human tissues. J. Virol. 78, 6381–6388 (2004).
Google Scholar
-
Yao, Y. et al. Variants of the adeno-associated virus serotype 9 with enhanced penetration of the blood–brain barrier in rodents and primates. Nat. Biomed. Eng. 6, 1257–1271 (2022). This paper uses a rational design approach to identify two new AAV9 variants that can cross the BBB.
Google Scholar
-
Kumar, S. R. et al. Multiplexed Cre-dependent selection yields systemic AAVs for targeting distinct brain cell types. Nat. Methods 17, 541–550 (2020).
Google Scholar
-
Goertsen, D. et al. AAV capsid variants with brain-wide transgene expression and decreased liver targeting after intravenous delivery in mouse and marmoset. Nat. Neurosci. 25, 106–115 (2022).
Google Scholar
-
Chan, K. Y. et al. Engineered AAVs for efficient noninvasive gene delivery to the central and peripheral nervous systems. Nat. Neurosci. 20, 1172–1179 (2017).
Google Scholar
-
Chen, X. et al. Engineered AAVs for non-invasive functional transgene expression in rodent and non-human primate central and peripheral nervous systems. Neuron 110, 2242–2257 (2022).
Google Scholar
-
Meyer, N. L. & Chapman, M. S. Adeno-associated virus (AAV) cell entry: structural insights. Trends Microbiol. 30, 432–451 (2021).
Google Scholar
-
Summerford, C. & Samulski, R. J. Membrane-associated heparan sulfate proteoglycan is a receptor for adeno-associated virus type 2 virions. J. Virol. 72, 1438–1445 (1998).
Google Scholar
-
Kaludov, N., Brown, K. E., Walters, R. W., Zabner, J. & Chiorini, J. A. Adeno-associated virus serotype 4 (AAV4) and AAV5 both require sialic acid binding for hemagglutination and efficient transduction but differ in sialic acid linkage specificity. J. Virol. 75, 6884–6893 (2001).
Google Scholar
-
Wu, Z., Miller, E., Agbandje-McKenna, M. & Samulski, R. J. α2,3 and α2,6 N-linked sialic acids facilitate efficient binding and transduction by adeno-associated virus types 1 and 6. J. Virol. 80, 9093–9103 (2006).
Google Scholar
-
Shen, S., Bryant, K. D., Brown, S. M., Randell, S. H. & Asokan, A. Terminal N-linked galactose is the primary receptor for adeno-associated virus 9. J. Biol. Chem. 286, 13532–13540 (2011).
Google Scholar
-
Riyad, J. M. & Weber, T. Intracellular trafficking of adeno-associated virus (AAV) vectors: challenges and future directions. Gene Ther. 28, 683–696 (2021).
Google Scholar
-
Penaud-Budloo, M. et al. Adeno-associated virus vector genomes persist as episomal chromatin in primate muscle. J. Virol. 82, 7875–7885 (2008).
Google Scholar
-
Dalwadi, D. A. et al. AAV integration in human hepatocytes. Mol. Ther. 29, 2898–2909 (2021).
Google Scholar
-
McCarty, D. M., Monahan, P. E. & Samulski, R. J. Self-complementary recombinant adeno-associated virus (scAAV) vectors promote efficient transduction independently of DNA synthesis. Gene Ther. 8, 1248–1254 (2001).
Google Scholar
-
McCarty, D. M. et al. Adeno-associated virus terminal repeat (TR) mutant generates self-complementary vectors to overcome the rate-limiting step to transduction in vivo. Gene Ther. 10, 2112–2118 (2003).
Google Scholar
-
Chen, X. et al. Biodistribution of adeno-associated virus gene therapy following cerebrospinal fluid-directed administration. Hum. Gene Ther. 34, 94–111 (2023). This paper conducts a comprehensive literature review to compare the biodistribution of AAV from different intra-CSF administration routes.
Google Scholar
-
Bharucha-Goebel, D. et al. O.10 First-in-human intrathecal gene transfer study for giant axonal neuropathy: preliminary review of long-term efficacy and safety. Neuromuscul. Disord. 32, S94 (2022).
Google Scholar
-
Bailey, R. M., Armao, D., Nagabhushan Kalburgi, S. & Gray, S. J. Development of intrathecal AAV9 gene therapy for giant axonal neuropathy. Mol. Ther. Methods Clin. Dev. 9, 160–171 (2018). This paper is the first study to show the safety and efficacy of using intrathecal lumbar administration route for delivering gene therapy to treat neurological disorders.
Google Scholar
-
Mendell, J. R. et al. Single-dose gene-replacement therapy for spinal muscular atrophy. N. Engl. J. Med. 377, 1713–1722 (2017). This paper reports clinical data from the clinical trial for SMA, the first gene therapy approved for a neurological disorder.
Google Scholar
-
Al-Zaidy, S. A. & Mendell, J. R. From clinical trials to clinical practice: practical considerations for gene replacement therapy in SMA type 1. Pediatr. Neurol. 100, 3–11 (2019).
Google Scholar
-
Burghes, A. H. & Beattie, C. E. Spinal muscular atrophy: why do low levels of survival motor neuron protein make motor neurons sick? Nat. Rev. Neurosci. 10, 597–609 (2009).
Google Scholar
-
Hinderer, C. et al. Severe toxicity in nonhuman primates and piglets following high-dose intravenous administration of an adeno-associated virus vector expressing human SMN. Hum. Gene Ther. 29, 285–298 (2018).
Google Scholar
-
Niethammer, M. et al. Gene therapy reduces Parkinson’s disease symptoms by reorganizing functional brain connectivity. Sci. Transl. Med. 10, eaau0713 (2018).
Google Scholar
-
Ciesielska, A. et al. Anterograde axonal transport of AAV2–GDNF in rat basal ganglia. Mol. Ther. 19, 922–927 (2011).
Google Scholar
-
Okada, S. & O’Brien, J. S. Generalized gangliosidosis: β-galactosidase deficiency. Science 160, 1002–1004 (1968).
Google Scholar
-
Gray-Edwards, H. L. et al. Novel biomarkers of human GM1 gangliosidosis reflect the clinical efficacy of gene therapy in a feline model. Mol. Ther. 25, 892–903 (2017).
Google Scholar
-
Gross, A. L. et al. Intravenous delivery of adeno-associated viral gene therapy in feline GM1 gangliosidosis. Brain 145, 655–669 (2022).
Google Scholar
-
Taghian, T. et al. Real-time MR tracking of AAV gene therapy with βgal-responsive MR probe in a murine model of GM1-gangliosidosis. Mol. Ther. Methods Clin. Dev. 23, 128–134 (2021).
Google Scholar
-
Mussche, S. et al. Restoration of cytoskeleton homeostasis after gigaxonin gene transfer for giant axonal neuropathy. Hum. Gene Ther. 24, 209–219 (2013).
Google Scholar
-
Fu, H. et al. Functional correction of neurological and somatic disorders at later stages of disease in MPS IIIA mice by systemic scAAV9–hSGSH gene delivery. Mol. Ther. Methods Clin. Dev. 3, 16036 (2016).
Google Scholar
-
Woodley, E. et al. Efficacy of a bicistronic vector for correction of sandhoff disease in a mouse model. Mol. Ther. Methods Clin. Dev. 12, 47–57 (2019).
Google Scholar
-
Rosenberg, J. B. et al. AAVrh.10-mediated APOE2 central nervous system gene therapy for APOE4-associated Alzheimer’s disease. Hum. Gene Ther. Clin. Dev. 29, 24–47 (2018).
Google Scholar
-
Flotte, T. R. et al. AAV gene therapy for Tay–Sachs disease. Nat. Med. 28, 251–259 (2022). This paper summarizes clinical findings from the first AAV gene therapy clinical trial treating two children with Tay–Sachs disease.
Google Scholar
-
Taghian, T. et al. A safe and reliable technique for CNS delivery of AAV vectors in the cisterna magna. Mol. Ther. 28, 411–421 (2020).
Google Scholar
-
Flotte, T. R. et al. Phase 1/2 clinical trial of combined bilateral intrathalamic/intracisternal/intrathecal delivery of a rAAVrh8 vector in infantile and juvenile Tay-Sachs and sandhoff disease: report of ongoing studies. In ESGCT 29th Annual Congress in collaboration with BSGCT Edinburgh, UK, October 11–14, 2022 Abstracts Vol. 33, A71 (Mary Ann Liebert, 2022).
-
Garcia-Sanz, P., Aerts, J. M. F. G. & Moratalla, R. The role of cholesterol in α-synuclein and lewy body pathology in GBA1 Parkinson’s disease. Mov. Disord. 36, 1070–1085 (2021).
Google Scholar
-
Li, D., Zhang, J. & Liu, Q. Brain cell type-specific cholesterol metabolism and implications for learning and memory. Trends Neurosci. 45, 401–414 (2022).
Google Scholar
-
Sucunza, D. et al. Glucocerebrosidase gene therapy induces α-synuclein clearance and neuroprotection of midbrain dopaminergic neurons in mice and macaques. Int. J. Mol. Sci. 22, 4825 (2021).
Google Scholar
-
Sheehan, P. et al. PR001 gene therapy improved phenotypes in models of Parkinson’s disease with GBA1 mutation: molecular and cell biology/endosomal–lysosomal dysfunction. Alzheimer’s Dement. 16, e043614 (2020).
Google Scholar
-
Tardieu, M. et al. Intracerebral administration of adeno-associated viral vector serotype rh.10 carrying human SGSH and SUMF1 cDNAs in children with mucopolysaccharidosis type IIIA disease: results of a phase I/II trial. Hum. Gene Ther. 25, 506–516 (2014).
Google Scholar
-
Tardieu, M. et al. Intracerebral gene therapy in children with mucopolysaccharidosis type IIIB syndrome: an uncontrolled phase 1/2 clinical trial. Lancet Neurol. 16, 712–720 (2017).
Google Scholar
-
Gougeon, M.-L. et al. Cell-mediated immunity to NAGLU transgene following intracerebral gene therapy in children with mucopolysaccharidosis type IIIB syndrome. Front. Immunol. 12, 655478 (2021).
Google Scholar
-
Sondhi, D. et al. Slowing late infantile Batten disease by direct brain parenchymal administration of a rh.10 adeno-associated virus expressing CLN2. Sci. Transl. Med. 12, eabb5413 (2020).
Google Scholar
-
Castle, M. J. et al. Postmortem analysis in a clinical trial of AAV2-NGF gene therapy for Alzheimer’s disease identifies a need for improved vector delivery. Hum. Gene Ther. 31, 415–422 (2020).
Google Scholar
-
Kantor, B., McCown, T., Leone, P. & Gray, S. J. Clinical applications involving CNS gene transfer. Adv. Genet. 87, 71–124 (2014).
Google Scholar
-
Hyland, K. & Clayton, P. Aromatic amino acid decarboxylase deficiency in twins. J. Inherit. Metab. Dis. 13, 301–304 (1990).
Google Scholar
-
Tai, C.-H. et al. Long-term efficacy and safety of eladocagene exuparvovec in patients with AADC deficiency. Mol. Ther. 30, 509–518 (2022). This paper summarizes the clinical findings from 26 patients with AADC deficiency receiving AAV gene therapy treatment through IPa administration, which led to the approval of this treatment by the EMA.
Google Scholar
-
Baek, R. C. et al. AAV-mediated gene delivery in adult GM1-gangliosidosis mice corrects lysosomal storage in CNS and improves survival. PLoS ONE 5, e13468 (2010).
Google Scholar
-
Cearley, C. N. & Wolfe, J. H. A single injection of an adeno-associated virus vector into nuclei with divergent connections results in widespread vector distribution in the brain and global correction of a neurogenetic disease. J. Neurosci. 27, 9928–9940 (2007).
Google Scholar
-
Pearson, T. S. et al. Gene therapy for aromatic l-amino acid decarboxylase deficiency by MR-guided direct delivery of AAV2–AADC to midbrain dopaminergic neurons. Nat. Commun. 12, 4251 (2021). This paper summarizes clinical findings from the first AAV gene therapy clinical trial using a magnetic resonance-guided IPa gene delivery approach.
Google Scholar
-
Salegio, E. A. et al. Feasibility of targeted delivery of AAV5–GFP into the cerebellum of nonhuman primates following a single convection-enhanced delivery infusion. Hum. Gene Ther. 33, 86–93 (2022).
Google Scholar
-
Yazdan-Shahmorad, A. et al. Widespread optogenetic expression in macaque cortex obtained with MR-guided, convection enhanced delivery (CED) of AAV vector to the thalamus. J. Neurosci. Methods 293, 347–358 (2018).
Google Scholar
-
Deverman, B. E., Ravina, B. M., Bankiewicz, K. S., Paul, S. M. & Sah, D. W. Y. Gene therapy for neurological disorders: progress and prospects. Nat. Rev. Drug. Discov. 17, 641 (2018).
Google Scholar
-
Ravina, B. et al. Intraputaminal AADC gene therapy for advanced Parkinson’s disease: interim results of a phase 1b trial [abstract]. Hum. Gene Ther. 28, OR12 (2017).
-
Davidson, B. L. et al. Recombinant adeno-associated virus type 2, 4, and 5 vectors: transduction of variant cell types and regions in the mammalian central nervous system. Proc. Natl Acad. Sci. USA 97, 3428–3432 (2000).
Google Scholar
-
Christine, C. W. et al. Safety of AADC gene therapy for moderately advanced Parkinson disease: three-year outcomes from the PD-1101 trial. Neurology 98, e40–e50 (2022).
Google Scholar
-
Luo, J. et al. Subthalamic GAD gene therapy in a Parkinson’s disease rat model. Science 298, 425–429 (2002).
Google Scholar
-
Niethammer, M. et al. Long-term follow-up of a randomized AAV2–GAD gene therapy trial for Parkinson’s disease. JCI Insight 2, e90133 (2017).
Google Scholar
-
Rocco, M. T. et al. Long-term safety of MRI-guided administration of AAV2–GDNF and gadoteridol in the putamen of individuals with Parkinson’s disease. Mol. Ther. 30, 3632–3638 (2022).
Google Scholar
-
Bartus, R. T. et al. Advancing neurotrophic factors as treatments for age-related neurodegenerative diseases: developing and demonstrating “clinical proof-of-concept” for AAV–neurturin (CERE-120) in Parkinson’s disease. Neurobiol. Aging 34, 35–61 (2013).
Google Scholar
-
Bartus, R. T. et al. Safety/feasibility of targeting the substantia nigra with AAV2–neurturin in Parkinson patients. Neurology 80, 1698–1701 (2013).
Google Scholar
-
Gray, S. J., Woodard, K. T. & Samulski, J. R. Viral vectors and delivery strategies for CNS gene therapy. Ther. Deliv. 1, 517–534 (2010).
Google Scholar
-
Heller, G. J. et al. Waning efficacy in a long-term AAV-mediated gene therapy study in the murine model of Krabbe disease. Mol. Ther. 29, 1883–1902 (2021).
Google Scholar
-
Chen, X. et al. AAV9/MFSD8 gene therapy is effective in preclinical models of neuronal ceroid lipofuscinosis type 7 disease. J. Clin. Invest. 132, e146286 (2022). This paper presents a case showing how a preclinical study is translated into a clinical trial, with all the data that are required for an investigational new drug (IND) application included.
Google Scholar
-
Kishimoto, T. K. & Samulski, R. J. Addressing high dose AAV toxicity—‘one and done’or ‘slower and lower’? Expert. Opin. Biol. Ther. 22, 1067–1071 (2022).
Google Scholar
-
Gray, S. J., Nagabhushan Kalburgi, S., McCown, T. J. & Jude Samulski, R. Global CNS gene delivery and evasion of anti-AAV-neutralizing antibodies by intrathecal AAV administration in non-human primates. Gene Ther. 20, 450–459 (2013).
Google Scholar
-
Samaranch, L. et al. Adeno-associated virus serotype 9 transduction in the central nervous system of nonhuman primates. Hum. Gene Ther. 23, 382–389 (2012).
Google Scholar
-
Kondratov, O. et al. A comprehensive study of a 29-capsid AAV library in a non-human primate central nervous system. Mol. Ther. 29, 2806–2820 (2021). This paper compares the biodistribution of 29 different AAV serotypes in the CNS of NHPs when administered through IPa and intra-CSF administration routes.
Google Scholar
-
Gray, S. J. Timing of gene therapy interventions: the earlier, the better. Mol. Ther. 24, 1017–1018 (2016).
Google Scholar
-
Rashnonejad, A. et al. Fetal gene therapy using a single injection of recombinant AAV9 rescued SMA phenotype in mice. Mol. Ther. 27, 2123–2133 (2019).
Google Scholar
-
Lowes, L. P. et al. Impact of age and motor function in a phase 1/2A study of infants with SMA type 1 receiving single-dose gene replacement therapy. Pediatr. Neurol. 98, 39–45 (2019).
Google Scholar
-
Chu, W. S. & Ng, J. Immunomodulation in administration of rAAV: preclinical and clinical adjuvant pharmacotherapies. Front. Immunol. 12, 858 (2021).
Google Scholar
-
Barnes, C., Scheideler, O. & Schaffer, D. Engineering the AAV capsid to evade immune responses. Curr. Opin. Biotech. 60, 99–103 (2019).
Google Scholar
-
Bertolini, T. B. et al. Effect of CpG depletion of vector genome on CD8+ T cell responses in AAV gene therapy. Front. Immunol. 12, 672449 (2021).
Google Scholar
-
Wright, J. F. Codon modification and PAMPs in clinical AAV vectors: the tortoise or the hare? Mol. Ther. 28, 701–703 (2020).
Google Scholar
-
Pan, X. et al. Rational engineering of a functional CpG-free ITR for AAV gene therapy. Gene Ther. 29, 333–345 (2022).
Google Scholar
-
Muhuri, M. et al. Overcoming innate immune barriers that impede AAV gene therapy vectors. J. Clin. Invest. 131, e143780 (2021).
Google Scholar
-
Kofoed, R. H. et al. Transgene distribution and immune response after ultrasound delivery of rAAV9 and PHP.B to the brain in a mouse model of amyloidosis. Mol. Ther. Methods Clin. Dev. 23, 390–405 (2021).
Google Scholar
-
Ciesielska, A. et al. Cerebral infusion of AAV9 vector-encoding non-self proteins can elicit cell-mediated immune responses. Mol. Ther. 21, 158–166 (2013).
Google Scholar
-
Hadaczek, P. et al. Transduction of nonhuman primate brain with adeno-associated virus serotype 1: vector trafficking and immune response. Hum. Gene Ther. 20, 225–237 (2009).
Google Scholar
-
Samaranch, L. et al. AAV9-mediated expression of a non-self protein in nonhuman primate central nervous system triggers widespread neuroinflammation driven by antigen-presenting cell transduction. Mol. Ther. 22, 329–337 (2014). This paper is the first to demonstrate that using AAV to deliver a non-self protein, but not a self protein, may trigger neural immune responses in NHPs.
Google Scholar
-
Ramsingh, A. I. et al. Sustained AAV9-mediated expression of a non-self protein in the CNS of non-human primates after immunomodulation. PLoS ONE 13, e0198154 (2018).
Google Scholar
-
Ling, Q., Rioux, M., Hu, Y., Lee, M. & Gray, S. J. Adeno-associated viral vector serotype 9-based gene replacement therapy for SURF1-related leigh syndrome. Mol. Ther. Methods Clin. Dev. 23, 158–168 (2021).
Google Scholar
-
Dell’Agnello, C. et al. Increased longevity and refractoriness to Ca2+-dependent neurodegeneration in Surf1 knockout mice. Hum. Mol. Genet. 16, 431–444 (2007).
Google Scholar
-
Quadalti, C. et al. SURF1 knockout cloned pigs: early onset of a severe lethal phenotype. Biochim. Biophys. Acta 1864, 2131–2142 (2018).
Google Scholar
-
Bradbury, A. M. et al. Krabbe disease successfully treated via monotherapy of intrathecal gene therapy. J. Clin. Invest. 130, 4906–4920 (2020).
Google Scholar
-
Karumuthil‐Melethil, S. et al. Intrathecal administration of AAV/GALC vectors in 10–11‐day‐old twitcher mice improves survival and is enhanced by bone marrow transplant. J. Neurosci. Res. 94, 1138–1151 (2016).
Google Scholar
-
Li, M. & Belmonte, I.J.C. Organoids — preclinical models of human disease. N. Engl. J. Med. 380, 569–579 (2019).
Google Scholar
-
Gorman, G. S. et al. Mitochondrial diseases. Nat. Rev. Dis. Prim. 2, 1–22 (2016).
-
Reynaud-Dulaurier, R. et al. Gene replacement therapy provides benefit in an adult mouse model of Leigh syndrome. Brain 143, 1686–1696 (2020).
Google Scholar
-
Silva-Pinheiro, P., Cerutti, R., Luna-Sanchez, M., Zeviani, M. & Viscomi, C. A single intravenous injection of AAV–PHP.B–hNDUFS4 ameliorates the phenotype of Ndufs4−/− mice. Mol. Ther. Methods Clin. Dev. 17, 1071–1078 (2020).
Google Scholar
-
Pereira, C. V. et al. Myopathy reversion in mice after restauration of mitochondrial complex I. EMBO Mol. Med. 12, e10674 (2020).
Google Scholar
-
Meo, I. D., Marchet, S., Lamperti, C., Zeviani, M. & Viscomi, C. AAV9-based gene therapy partially ameliorates the clinical phenotype of a mouse model of Leigh syndrome. Gene Ther. 24, 661–667 (2017).
Google Scholar
-
Abrams, A. J. et al. Mutations in SLC25A46, encoding a UGO1-like protein, cause an optic atrophy spectrum disorder. Nat. Genet. 47, 926–932 (2015).
Google Scholar
-
Janer, A. et al. SLC25A46 is required for mitochondrial lipid homeostasis and cristae maintenance and is responsible for Leigh syndrome. EMBO Mol. Med. 8, 1019–1038 (2016).
Google Scholar
-
Charlesworth, G. et al. SLC25A46 mutations underlie progressive myoclonic ataxia with optic atrophy and neuropathy. Mov. Disord. 31, 1249–1251 (2016).
Google Scholar
-
Hammer, M. B. et al. SLC25A46 mutations associated with autosomal recessive cerebellar ataxia in North African families. Neurodegener. Dis. 17, 208–212 (2017).
Google Scholar
-
Yang, L. et al. Systemic administration of AAV–Slc25a46 mitigates mitochondrial neuropathy in Slc25a46−/− mice. Hum. Mol. Genet. 29, 649–661 (2020).
Google Scholar
-
Vila-Julia, F. et al. Efficacy of adeno-associated virus gene therapy in a MNGIE murine model enhanced by chronic exposure to nucleosides. EBioMedicine 62, 103133 (2020).
Google Scholar
-
Di Meo, I. et al. Effective AAV‐mediated gene therapy in a mouse model of ethylmalonic encephalopathy. EMBO Mol. Med. 4, 1008–1014 (2012).
Google Scholar
-
Chadderton, N. et al. Intravitreal delivery of AAV–NDI1 provides functional benefit in a murine model of Leber hereditary optic neuropathy. Eur. J. Hum. Genet. 21, 62–68 (2013).
Google Scholar
-
Koilkonda, R. D. et al. Safety and effects of the vector for the Leber hereditary optic neuropathy gene therapy clinical trial. JAMA Ophthalmol. 132, 409–420 (2014).
Google Scholar
-
Torres-Torronteras, J. et al. Long-term sustained effect of liver-targeted adeno-associated virus gene therapy for mitochondrial neurogastrointestinal encephalomyopathy. Hum. Gene Ther. 29, 708–718 (2018).
Google Scholar
-
Fountain, M. D. & Schaaf, C. P. Prader–Willi syndrome and Schaaf–Yang syndrome: neurodevelopmental diseases intersecting at the MAGEL2 gene. Diseases 4, 2 (2016).
Google Scholar
-
Queen, N. J. et al. Hypothalamic AAV–BDNF gene therapy improves metabolic function and behavior in the Magel2-null mouse model of Prader–Willi syndrome. Mol. Ther. Methods Clin. Dev. 27, 131–148 (2022).
Google Scholar
-
Zeier, Z. et al. Fragile X mental retardation protein replacement restores hippocampal synaptic function in a mouse model of fragile X syndrome. Gene Ther. 16, 1122–1129 (2009).
Google Scholar
-
Arsenault, J. et al. FMRP expression levels in mouse central nervous system neurons determine behavioral phenotype. Hum. Gene Ther. 27, 982–996 (2016).
Google Scholar
-
Gholizadeh, S., Arsenault, J., Xuan, I. C. Y., Pacey, L. K. & Hampson, D. R. Reduced phenotypic severity following adeno-associated virus-mediated Fmr1 gene delivery in fragile X mice. Neuropsychopharmacology 39, 3100–3111 (2014).
Google Scholar
-
Turner, T. J., Zourray, C., Schorge, S. & Lignani, G. Recent advances in gene therapy for neurodevelopmental disorders with epilepsy. J. Neurochem. 157, 229–262 (2021).
Google Scholar
-
Davidson, B. L. et al. Gene-based therapeutics for rare genetic neurodevelopmental psychiatric disorders. Mol. Ther. 30, 2416–2428 (2022). This paper summarizes the discussions and presentations of ‘Gene-Based Therapeutics for Rare Genetic Neurodevelopmental Psychiatric Disorders’, a National Institute of Mental Health-sponsored workshop held in January 2021.
Google Scholar
-
Prabhakar, S. et al. Long-term therapeutic efficacy of intravenous AAV-mediated hamartin replacement in mouse model of tuberous sclerosis type 1. Mol. Ther. Methods Clin. Dev. 15, 18–26 (2019).
Google Scholar
-
Gao, Y. et al. Gene replacement ameliorates deficits in mouse and human models of cyclin-dependent kinase-like 5 disorder. Brain 143, 811–832 (2020).
Google Scholar
-
Taysha Gene Therapies Announces Initiation of Clinical Development of TSHA-102 in Rett Syndrome. Taysha https://ir.tayshagtx.com/news-releases/news-release-details/taysha-gene-therapies-announces-initiation-clinical-0 (2022).
-
Sinnett, S. E., Boyle, E., Lyons, C. & Gray, S. J. Engineered microRNA-based regulatory element permits safe high-dose mini MECP2 gene therapy in Rett mice. Brain 144, 3005–3019 (2021). This paper shows the development of a self-regulatory gene therapy approach using endogenous miRNAs.
Google Scholar
-
Luoni, M. et al. Whole brain delivery of an instability-prone Mecp2 transgene improves behavioral and molecular pathological defects in mouse models of Rett syndrome. eLife 9, e52629 (2020).
Google Scholar
-
Heeroma, J. H. et al. Episodic ataxia type 1 mutations differentially affect neuronal excitability and transmitter release. Dis. Model. Mech. 2, 612–619 (2009).
Google Scholar
-
Qiu, Y. et al. On-demand cell-autonomous gene therapy for brain circuit disorders. Science 378, 523–532 (2022).
Google Scholar
-
Judson, M. C. et al. Dual-isoform hUBE3A gene transfer improves behavioral and seizure outcomes in Angelman syndrome model mice. JCI Insight 6, e144712 (2021).
Google Scholar
-
Ogiwara, I. et al. Nav1.1 localizes to axons of parvalbumin-positive inhibitory interneurons: a circuit basis for epileptic seizures in mice carrying an Scn1a gene mutation. J. Neurosci. 27, 5903–5914 (2007).
Google Scholar
-
Tanenhaus, A. et al. Cell-selective AAV-mediated SCN1A gene regulation therapy rescues mortality and seizure phenotypes in a dravet syndrome mouse model and is well tolerated in non-human primates. Hum. Gene Ther. 33, 579–597 (2022).
Google Scholar
-
Howden, S., Voullaire, L. & Vadolas, J. The transient expression of mRNA coding for Rep protein from AAV facilitates targeted plasmid integration. J. Gene Med. 10, 42–50 (2008).
Google Scholar
-
Dalwadi, D. A. et al. Liver injury increases the incidence of HCC following AAV gene therapy in mice. Mol. Ther. 29, 680–690 (2021).
Google Scholar
-
Donsante, A. et al. AAV vector integration sites in mouse hepatocellular carcinoma. Science 317, 477–477 (2007).
Google Scholar
-
Nguyen, G. N. et al. A long-term study of AAV gene therapy in dogs with hemophilia A identifies clonal expansions of transduced liver cells. Nat. Biotechnol. 39, 47–55 (2021).
Google Scholar
-
Day, J. W. et al. Clinical trial and postmarketing safety of onasemnogene abeparvovec therapy. Drug. Saf. 44, 1109–1119 (2021).
Google Scholar
-
Guillou, J. et al. Fatal thrombotic microangiopathy case following adeno-associated viral SMN gene therapy. Blood Adv. 6, 4266–4270 (2022).
Google Scholar
-
Hordeaux, J. et al. Adeno-associated virus-induced dorsal root ganglion pathology. Hum. Gene Ther. 31, 808–818 (2020). This paper analyses potential factors leading to DRG pathology from AAV gene therapy by compiling data from 33 non-clinical studies in NHPs.
Google Scholar
-
Buss, N. et al. Characterization of AAV-mediated dorsal root ganglionopathy. Mol. Ther. Methods Clin. Dev. 24, 342–354 (2022).
Google Scholar
-
Mueller, C. et al. SOD1 suppression with adeno-associated virus and microRNA in familial ALS. N. Engl. J. Med. 383, 151–158 (2020).
Google Scholar
-
Hordeaux, J. et al. microRNA-mediated inhibition of transgene expression reduces dorsal root ganglion toxicity by AAV vectors in primates. Sci. Transl. Med. 12, eaba9188 (2020).
Google Scholar
-
Kayani, S, et al. Preliminary safety data of a phase 1 first in-human clinical trial support the use of high dose intrathecal AAV9/CLN7 for the treatment of patients with CLN7 disease. Mol. Genet. Metabol. 135, S65 (2022).
Google Scholar
-
Rosenberg, J. B. et al. Safety of direct intraparenchymal AAVrh.10-mediated central nervous system gene therapy for metachromatic leukodystrophy. Hum. Gene Ther. 32, 563–580 (2021).
Google Scholar
-
Agbandje-McKenna, M. & Kleinschmidt, J. AAV capsid structure and cell interactions. Methods Mol. Biol. 807, 47–92 (2011).
Google Scholar
-
Salganik, M. et al. Adeno-associated virus capsid proteins may play a role in transcription and second-strand synthesis of recombinant genomes. J. Virol. 88, 1071–1079 (2014).
Google Scholar
-
Kanaan, N. M. et al. Rationally engineered AAV capsids improve transduction and volumetric spread in the CNS. Mol. Ther. Nucleic Acids 8, 184–197 (2017).
Google Scholar
-
Chan, Y. K. et al. Engineering adeno-associated viral vectors to evade innate immune and inflammatory responses. Sci. Transl. Med. 13, eabd3438 (2021).
Google Scholar
-
Deverman, B. E. et al. Cre-dependent selection yields AAV variants for widespread gene transfer to the adult brain. Nat. Biotechnol. 34, 204–209 (2016).
Google Scholar
-
Hordeaux, J. et al. The neurotropic properties of AAV–PHP.B are limited to C57BL/6J mice. Mol. Ther. 26, 664–668 (2018). This paper demonstrates that AAV vectors can show different tropisms in different species.
Google Scholar
-
Matsuzaki, Y. et al. Intravenous administration of the adeno-associated virus–PHP.B capsid fails to upregulate transduction efficiency in the marmoset brain. Neurosci. Lett. 665, 182–188 (2018).
Google Scholar
-
Huang, Q. et al. Delivering genes across the blood–brain barrier: LY6A, a novel cellular receptor for AAV-PHP.B capsids. PLoS ONE 14, e0225206 (2019).
Google Scholar
-
Lin, R. et al. Directed evolution of adeno-associated virus for efficient gene delivery to microglia. Nat. Methods 19, 976–985 (2022).
Google Scholar
-
Beharry, A. et al. The AAV9 variant capsid AAV-F mediates widespread transgene expression in nonhuman primate spinal cord after intrathecal administration. Hum. Gene Ther. 33, 61–75 (2022).
Google Scholar
-
Stanton, A. C. et al. Systemic administration of novel engineered AAV capsids facilitates enhanced transgene expression in the macaque CNS. Medicine 4, 31–50 (2022).
Google Scholar
-
Mich, J. K. et al. Functional enhancer elements drive subclass-selective expression from mouse to primate neocortex. Cell Rep. 34, 108754 (2021).
Google Scholar
-
Powell, S. K., Rivera-Soto, R. & Gray, S. J. Viral expression cassette elements to enhance transgene target specificity and expression in gene therapy. Discov. Med. 19, 49–57 (2015).
Google Scholar
-
Jackson, K. L., Dayton, R. D., Deverman, B. E. & Klein, R. L. Better targeting, better efficiency for wide-scale neuronal transduction with the synapsin promoter and AAV-PHP.B. Front. Mol. Neurosci. 9, 116 (2016).
Google Scholar
-
Xie, J. et al. microRNA-regulated, systemically delivered rAAV9: a step closer to CNS-restricted transgene expression. Mol. Ther. 19, 526–535 (2011).
Google Scholar
-
Sidonio, R. F. Jr et al. Discussing investigational AAV gene therapy with hemophilia patients: a guide. Blood Rev. 47, 100759 (2021).
Google Scholar
-
Athey, J. et al. A new and updated resource for codon usage tables. BMC Bioinforma. 18, 391 (2017).
Google Scholar
-
Mauro, V. P. & Chappell, S. A. A critical analysis of codon optimization in human therapeutics. Trends Mol. Med. 20, 604–613 (2014).
Google Scholar
-
Agashe, D., Martinez-Gomez, N. C., Drummond, D. A. & Marx, C. J. Good codons, bad transcript: large reductions in gene expression and fitness arising from synonymous mutations in a key enzyme. Mol. Biol. Evol. 30, 549–560 (2013).
Google Scholar
-
Spencer, P. S., Siller, E., Anderson, J. F. & Barral, J. M. Silent substitutions predictably alter translation elongation rates and protein folding efficiencies. J. Mol. Biol. 422, 328–335 (2012).
Google Scholar
-
Tsai, C.-J. et al. Synonymous mutations and ribosome stalling can lead to altered folding pathways and distinct minima. J. Mol. Biol. 383, 281–291 (2008).
Google Scholar
-
Zhou, J.-h et al. The effects of the synonymous codon usage and tRNA abundance on protein folding of the 3C protease of foot-and-mouth disease virus. Infect. Genet. Evol. 16, 270–274 (2013).
Google Scholar
-
Dominguez, E. et al. Intravenous scAAV9 delivery of a codon-optimized SMN1 sequence rescues SMA mice. Hum. Mol. Genet. 20, 681–693 (2011).
Google Scholar
-
Hordeaux, J. et al. Efficacy and safety of a Krabbe disease gene therapy. Hum. Gene Ther. 33, 499–517 (2022).
Google Scholar
-
Chand, D. et al. Hepatotoxicity following administration of onasemnogene abeparvovec (AVXS-101) for the treatment of spinal muscular atrophy. J. Hepatol. 74, 560–566 (2021).
Google Scholar
-
Feldman, A. G. et al. Subacute liver failure following gene replacement therapy for spinal muscular atrophy type 1. J. Pediatr. 225, 252–258 (2020).
Google Scholar
-
Hordeaux, J. et al. Toxicology study of intra-cisterna magna adeno-associated virus 9 expressing iduronate-2-sulfatase in rhesus macaques. Mol. Ther. Methods Clin. Dev. 10, 68–78 (2018).
Google Scholar
-
Li, Y. et al. Enhanced efficacy and increased long-term toxicity of CNS-directed, AAV-based combination therapy for Krabbe disease. Mol. Ther. 29, 691–701 (2021).
Google Scholar
-
Boudreau, R. L., Spengler, R. M. & Davidson, B. L. Rational design of therapeutic siRNAs: minimizing off-targeting potential to improve the safety of RNAi therapy for Huntington’s disease. Mol. Ther. 19, 2169–2177 (2011).
Google Scholar
-
Boudreau, R. L., Martins, I. & Davidson, B. L. Artificial microRNAs as siRNA shuttles: improved safety as compared to shRNAs in vitro and in vivo. Mol. Ther. 17, 169–175 (2009).
Google Scholar
-
Franich, N. R. et al. AAV vector-mediated RNAi of mutant Huntingtin expression is neuroprotective in a novel genetic rat model of Huntington’s disease. Mol. Ther. 16, 947–956 (2008).
Google Scholar
-
Miniarikova, J. et al. AAV5–miHTT gene therapy demonstrates suppression of mutant huntingtin aggregation and neuronal dysfunction in a rat model of Huntington’s disease. Gene Ther. 24, 630–639 (2017).
Google Scholar
-
Meglio, M. uniQure receives DSMB recommendation to resume higher dosing of AMT-130 in huntington trial. Neurology Live https://www.neurologylive.com/view/uniqure-receives-dsmb-recommendation-resume-higher-dosing-amt-130-huntington-trial (2022).
-
Curtis, H. J., Seow, Y., Wood, M. J. & Varela, M. A. Knockdown and replacement therapy mediated by artificial mirtrons in spinocerebellar ataxia 7. Nucleic Acids Res. 45, 7870–7885 (2017).
Google Scholar
-
Gaj, T., Guo, J., Kato, Y., Sirk, S. J. & Barbas, C. F. III Targeted gene knockout by direct delivery of zinc-finger nuclease proteins. Nat. Methods 9, 805–807 (2012).
Google Scholar
-
Liu, J., Gaj, T., Patterson, J. T., Sirk, S. J. & Barbas, C. F. III Cell-penetrating peptide-mediated delivery of TALEN proteins via bioconjugation for genome engineering. PLoS ONE 9, e85755 (2014).
Google Scholar
-
Carroll, D. Genome engineering with zinc-finger nucleases. Genetics 188, 773–782 (2011).
Google Scholar
-
Yamaguchi, T. et al. Aspects of gene therapy products using current genome-editing technology in Japan. Hum. Gene Ther. 31, 1043–1053 (2020).
Google Scholar
-
Newby, G. A. & Liu, D. R. In vivo somatic cell base editing and prime editing. Mol. Ther. 29, 3107–3124 (2021).
Google Scholar
-
Abudayyeh, O. O. et al. A cytosine deaminase for programmable single-base RNA editing. Science 365, 382–386 (2019).
Google Scholar
-
Lubroth, P., Colasante, G. & Lignani, G. In vivo genome editing therapeutic approaches for neurological disorders: where are we in the translational pipeline? Front. Neurosci. 15, 632522 (2021).
Google Scholar
Author information
Authors and Affiliations
Contributions
All authors researched data for the article. S.J.G. and Q.L. contributed substantially to discussion of the content. Q.L. and J.H. wrote the article. All authors reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
S.J.G. has received royalty income from inventions discussed in the article, through licensing agreements with Neurogene, Asklepios Biopharmaceuticals, Taysha Gene Therapies and Abeona Therapeutics. A.B. has received royalty income from inventions discussed in the article from Axovant Gene Therapies and Neurogene. Q.L. has received royalty income from inventions discussed in the article from Taysha Gene Therapies.
Peer review
Peer review information
Nature Reviews Drug Discovery thanks Stephanie Schorge and Olivier Danos for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Glossary
- Capsid
-
The structural protein surrounding the genome of an encapsulated virus, including adeno-associated viruses (AAVs).
- Convection-enhanced delivery
-
An experimental gene therapy delivery technique that uses a catheter to insert a thin tube into the brain and applies pressure to deliver the vector.
- Cross-reactive immunological material
-
(CRIM). Typically refers to the presence of ‘self’ antigens by an individual, such that their immune system is tolerant to those antigens.
- Episome
-
A closed circular extrachromosomal DNA molecule formed from a viral genome that serves as a transcription template.
- Haploinsufficiency
-
When one copy of a gene is mutated, which leads to loss of function of the protein, only half the amount of functional protein is produced, and that is not enough to support normal cellular functions.
- Hepatotoxicity
-
Liver-related adverse effects usually indicated by increased aspartate aminotransferase and alanine aminotransferase levels, sometimes accompanied by thrombocytopenia and coagulopathy.
- Immediate early gene
-
A gene that is activated rapidly and transiently in response to a wide variety of cellular stimuli, such as neuronal activity.
- Intraparenchymal
-
Within the functional tissue of an organ, which in this Review refers to the brain.
- Kozak sequence
-
A nucleic acid motif for initiation of translation in vertebrates. The consensus sequence is GCCRCCAUGG, where R is a purine (A or G) and AUG is the initiation codon.
- Open reading frame
-
A start codon followed by a portion of in-frame DNA sequence that does not include a stop codon.
- Promoter
-
The upstream element to a gene that can control the timing and cell specificity of expression through the recruitment of transcriptional machinery.
- Serotype
-
A virus classification based on surface antigen expression and determined by immunological responses in host serum.
- Variant
-
Similar to serotype, a viral variant is classified according to surface antigen expression or other characteristics, but is not determined by immunological responses in host serum.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and Permissions
About this article
Cite this article
Ling, Q., Herstine, J.A., Bradbury, A. et al. AAV-based in vivo gene therapy for neurological disorders.
Nat Rev Drug Discov (2023). https://doi.org/10.1038/s41573-023-00766-7
-
Accepted: 06 July 2023
-
Published: 01 September 2023
-
DOI: https://doi.org/10.1038/s41573-023-00766-7