Congenital disorders
Function of a mutant ryanodine receptor (T4709M) linked to congenital myopathy
Abstract
Physiological muscle contraction requires an intact ligand gating mechanism of the ryanodine receptor 1 (RyR1), the Ca2+-release channel of the sarcoplasmic reticulum. Some mutations impair the gating and thus cause muscle disease. The RyR1 mutation T4706M is linked to a myopathy characterized by muscle weakness. Although, low expression of the T4706M RyR1 protein can explain in part the symptoms, little is known about the function RyR1 channels with this mutation. In order to learn whether this mutation alters channel function in a manner that can account for the observed symptoms, we examined RyR1 channels isolated from mice homozygous for the T4709M (TM) mutation at the single channel level. Ligands, including Ca2+, ATP, Mg2+ and the RyR inhibitor dantrolene were tested. The full conductance of the TM channel was the same as that of wild type (wt) channels and a population of partial open (subconductive) states were not observed. However, two unique sub-populations of TM RyRs were identified. One half of the TM channels exhibited high open probability at low (100 nM) and high (50 μM) cytoplasmic [Ca2+], resulting in Ca2+-insensitive, constitutively high Po channels. The rest of the TM channels exhibited significantly lower activity within the physiologically relevant range of cytoplasmic [Ca2+], compared to wt. TM channels retained normal Mg2+ block, modulation by ATP, and inhibition by dantrolene. Together, these results suggest that the TM mutation results in a combination of primary and secondary RyR1 dysfunctions that contribute to disease pathogenesis.
Introduction
Ryanodine receptor type 1 (RyR1) is the Ca2+ channel of the sarcoplasmic reticulum (SR) of the skeletal muscle. When RyR1 channels open under the control of an action potential in the sarcolemma, Ca2+ is released from the SR, which is used to drive muscle contraction. The communication between the sarcolemma and RyR1 is mediated by the dihydropyridine receptor (DHPR) in a voltage dependent manner through an allosteric mechanism, called excitation–contraction coupling (ECC)1, 2. RyR1 channels are regulated by a variety of ligands, including Ca2+, ATP and Mg2+3,4,5,6,7,8,9. In terms of regulation, the most relevant ligand is Ca2+, which activates RyR1 in the micromolar range and allows maximal channel activity at 50 µM6. The role of cytoplasmic Ca2+ in ECC is to open RyR channels that are not directly allosterically coupled to DHPRs, within a process termed Ca2+-induced Ca2+ release10, 11. In this way Ca2+ release amplifies the Ca2+ signal. Mg2+ inhibits RyR1 channels in the millimolar range by competing for the Ca2+ binding site8. ATP is one of the most effective agonists of RyR1, as it significantly activates the channel even in the absence of Ca2+ and can further enhance RyR1 channel open probability even in 50 µM Ca2+9.
Mutations impairing RyR function or its regulation cause a wide spectrum of muscle disorders, collectively called ryanopathies12. Among these, RyR1-related myopathies are often classified according to their specific histological presentation. Major histopathological groups of RYR1-related myopathies include central core disease (CCD), multiminicore disease, core-rod myopathy, and centronuclear myopathy. The patients suffer from mild, slowly- or non-progressive disabilities due to proximal muscle weakness and fatigue, low muscle tone and slow contraction. Symptoms also include respiratory muscle weakness, scoliosis, orthopedic deformities, pronounced facial weakness and dysmorphism, ptosis, ophthalmoparesis, exertional heat stroke, exertional myalgia and malignant hyperthermia susceptibility13, 14. Malignant hyperthermia is an idiosyncratic reaction to volatile anaesthetics (such as halothane and isoflurane) and succinylcholine. Susceptible patients exhibit muscle contractures all over the body when exposed to therapeutic concentrations of these trigger-drugs. Consequently, the body temperature rapidly increases, acidosis and hyperkalaemia develop, leading to an acute life-threatening condition unless the body is cooled down and dantrolene, an RyR1 inhibitor and muscle relaxant, is immediately administered15,16,17,18,19,20.
At the cellular level, RyR1 channels carrying certain MH causing mutations showed elevated basal activity when expressed in skeletal muscle fibres, indicating that there was a resting Ca2+ leak from the SR. Nevertheless, both the Ca2+ content of the SR and the resting myoplasmic Ca2+ concentration remained normal, indicating that the leak was low enough to be compensated by the SR Ca2+ pump. However, the threshold for activation of Ca2+ release by voltage and caffeine was significantly lower, suggesting that the mutations increased the sensitivity of RyR1 to activation by both endogenous (i.e. DHPR electromechanical uncoupling) and exogenous (caffeine) activators21,22,23. In contrast, RyR1 channels carrying other more severe CCD mutations showed a substantial elevation resting activity, leading to decompensated Ca2+ leak and consequent depletion of Ca2+ from the SR, resulting in attenuated Ca2+ release23, 24. Other mutations located within the RyR1 pore region result in a reduction in Ca2+ RyR1 permeation, and thus, a function uncoupling of excitation from SR Ca2+ release (termed “EC uncoupling”)23, 25. Apparently, the clinical symptoms can be explained by both of these mechanistic alterations in RyR1 function (RyR1 Ca2+ leak and EC uncoupling).
In some new cases of RyR1-related myopathies, pathologic SR Ca2+ leak was shown to be associated with low levels of the regulatory protein FKBP12 (calstabin) bound to the RyR126. FKBP12 stabilizes the closed state of the RyR1 channel and dissociation causes leaky RyR1 channels by promoting subconductive openings. Posttranslational RyR1 modifications, such as PKA mediated hyperphosphorylation, oxidation and S-nitrosylation were reported to dissociate FKBP12, and thus, increase RyR1 Ca2+ leak in certain muscle disorders27,28,29,30,31,32,33. On the other hand, some RyR1-related myopathy mutations were reported to affect RyR1 channel conductance, ligand (Ca2+, ATP) regulation or expression of the channel21, 34, 35.
Diagnosis of myopathies traditionally relies on the histological findings from a muscle biopsy36, but recently the diagnostic procedure is enhanced by the sequencing of the entire RyR1 gene. Owing to this technical development, the number of newly discovered mutations is increasing37,38,39. Both dominant and recessive forms of RyR1-related myopathies have been reported, with the recessive forms being often associated with a more severe clinical presentation40, 41. An example for such a mutation is T4706M. Despite the low incidence rate of these recessive mutations, their medical relevance may be significant, because some heterozygous cases occur together with epigenetic allele silencing of the wild type allele resulting in a pseudo-recessive phenotype, including severe facial and proximal weakness, scoliosis, opthalmoplegia and respiratory impairment42, 43. Homozygous T4709M knock-in RyR1 mice (equivalent to the T4706M in human RyR1) were generated and examined previously42. Homozygous TM mice exhibit mild muscle weakness, kyphosis, enhanced isoflurane sensitivity and reduced RyR1 expression (~ 30% reduction). Compound heterozygous mice with one TM allele and one frameshift (null) allele express a markedly lower level of RyR1 channels (~ 80% reduction) and exhibit an even more severe (post-natal lethal) myopathy. Importantly, both models are expected to result in only homotypic TM/TM channels, with a higher level of overall channel expression in muscle of homozygous TM/TM mice42.
TM mutation is located adjacent to the RyR1 caffeine binding site44. Prior studies conducted on recombinant RyR1 channels expressed and purified from Hek293 cells found that the homotypic TM RyR1 channels exhibited a normal low open probability in low Ca2+, but an increased sensitivity to activation by caffeine. However, the function of endogenous homotypic TM channels in skeletal muscle and the degree to which TM channel dysfunction contributes to muscle dysfunction is unclear42, 43. This question of homotypic T4706M RyR’s functionality is particularly important because reactivation of the wt silenced allele using DNA methyltransferase inhibitors appears to be a promising future therapeutic option to improve RyR1 expression, but improvement in muscle function can only be expected if RyR1 function is adequate43.
As homozygous TM/TM mice (TM) express a higher level of homotypic RyR1 channels, purified RyR1 channels from skeletal muscle of wt and homozygous TM mice in order to directly evaluate the impact of the T4706M mutation on RyR1 channel function at the single-channel level. Here, we characterized the unitary conductance, ligand (Ca2+, ATP, and Mg2+) sensitivity, and biophysical and pharmacological properties of single wt and homotypic T4709M RyR1 channels reconstituted in planar lipid bilayers.
Materials and methods
The methods were conducted in accordance with the ARRIVE guidelines. All methods were performed in accordance with the relevant guidelines and regulations. All animal studies were designed to minimize animal suffering and were approved by the University Committee on Animal Resources at the University of Rochester (UCAR2006-114E).
Materials
Phospholipids were obtained from Avanti Polar Lipids, Inc. (Alabaster, AL). All other chemicals, if not specified, were purchased from Sigma-Aldrich (St. Louis, MO).
Ryanodine receptor purification
13 g of skeletal muscle was collected from wild type (wt) and genetically modified homozygous mice carrying the patient-relevant T4709M mutation in RyR1 (equivalent to T4706M in human RyR1) (n = 10 for each genotype)42. Mice were euthanized by CO2 anaesthesia followed by cervical dislocation. Each step of the purification protocol was performed on ice or at 4 °C in the presence of protease inhibitors (pefabloc SC, aprotinin, leupeptin, benzamidine, pepstatin A and calpain inhibitor). The muscle samples pooled from 10 mice and were homogenised in: 100 mM NaCl, 20 mM EGTA, 20 mM Na-HEPES (pH = 7.5). Cell debris was pelleted at 3500 × g, for 35 min by using a laboratory centrifuge. SR microsomes were isolated by using differential centrifugation as follows. Crude microsomes were collected from the supernatant by centrifugation in a Ti45 rotor at 40,000 × g, for 30 min. To remove the actomyosin contamination the pellet was resuspended in: 600 mM KCl, 10 mM K-PIPES, 250 mM sucrose, 1 mM EGTA, 0.9 mM CaCl2 (pH = 7.0) and incubated for 1 h. Then, the microsomes were collected by centrifugation at 109,000 × g for 30 min, and the pellet was resuspended in: 300 mM sucrose, 10 mM K-PIPES (pH = 7.0), snap-frozen in liquid nitrogen, and stored at – 70 °C until the solubilisation step.
Microsomes were allowed to melt on ice and solubilized in: 1% CHAPS, 1 M NaCl, 100 μM EGTA, 150 μM CaCl2, 5 mM AMP, 0.45% phosphatidylcholine, 20 mM Na-PIPES (pH = 7.2) for 2 h. The samples were loaded onto a 10–28% linear sucrose gradient and centrifuged overnight at 90,000 × g in an SW27 rotor. RyR-containing fractions of the gradient were snap-frozen in liquid nitrogen and stored at − 70 °C in small aliquots45,46,47.
SDS-PAGE was used to verify the purified samples. 30 μl of each fraction (~ 400 μl) of the sucrose gradient was loaded in each well of a 10% linear gel. After electrophoresis, gels were stained with Coomassie Blue6. Images were converted to black and white and were not further processed. Full length gels are presented.
RyR reconstitution and single-channel current recording
Voltage-clamp measurements were performed on purified RyR1 channels incorporated into artificial planar lipid bilayers. Bilayers were formed across an aperture with a diameter of 200 μm drilled in the wall of a Delrin cap (Warner Instruments, Hamden, CT), which separated two chambers. The chambers were filled with a recording solution containing: 250 mM KCl, 50 μM CaCl2, 10 mM HEPES (pH = 7.2). Transmembrane voltages were reference to ground (trans-to-cis), allowing ionic currents of physiological direction through open RyR channels. The free [Ca2+] of the recording medium was adjusted using an EGTA stock solution.
The lipid mixture contained phosphatidylethanolamine, phosphatidylserine, and phosphatidylcholine (Avanti Polar Lipids, Alabaster, AL) in a ratio of 5:4:1 was dissolved in n-decane at a final concentration of 20 mg/mL. The bilayer currents were recorded in voltage-clamp mode using an AxoPatch 200 amplifier and pCLAMP 6.03 (Axon Instruments, Sunnyvale, CA) software. The holding potentials were as indicated in the text. The currents were filtered at 1 kHz through an eight-pole low-pass Bessel filter and digitized at 3 kHz45,46,47.
Statistics
Open probabilities (Po) were determined using the pClamp software suite (Molecular Devices, Sunnyvale, CA). Statistical analyses were performed in Origin 7.0 (OriginLab, Northampton, MA) and in Excel (Microsoft, Redmond, WA). Results were expressed as mean ± standard error (SE). Relative Po data were calculated by normalizing each data point to their own control. Statistical significance of differences was evaluated by using the independent two-sample t-test. Differences were considered significant when p was less than 0.05 (marked by *). The number of observations is displayed in the figure.
Results and discussion
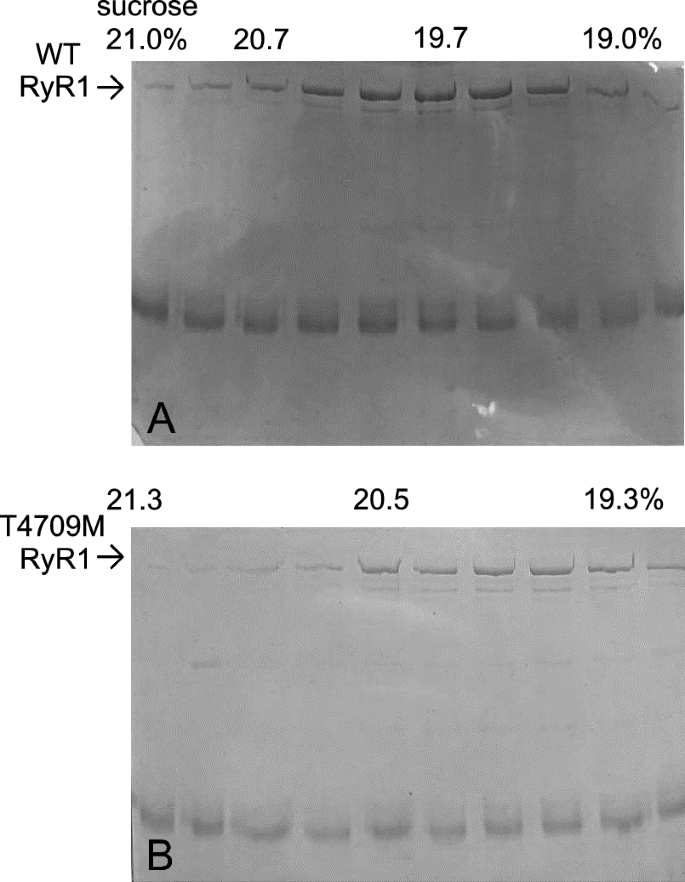
Skeletal muscle was collected from wt and homozygous T4709M (TM) RyR1 knock-in mice and RyRs were purified from the tissue using sucrose gradient ultracentrifugation. The resulting samples were verified for RyR1 content using SDS-PAGE. Small volumes of 10 different fractions, collected between the 18 and 21% sucrose concentration range were loaded on a gel and stained with Coomassie blue after electrophoresis. Consistent with prior reports, the RyR1 expression was lower in the case of TM muscle, but no evidence for fragmentation of the protein was found (Fig. 1).
SDS-PAGE of RyR1 samples purified from skeletal muscle tissue. (A) Samples of different sucrose gradient fractions of a RyR1 preparation from wt mice. The arrow indicates the band of the RyR1 protein. Sucrose concentrations (%) were as indicated. (B) Samples of a RyR1 preparation as in (A) from a homozygous T4709M RyR1 mice.
RyR channels from sucrose fractions ~ 19–21% were reconstituted in planar lipid bilayers. The single-channel conductance in 250 mM KCl recording solution not significantly different between the two groups: 832 ± 6.9 pS for wt channels and 824 ± 4.1 pS for TM channels (Fig. 2A). Current transitions, partial openings of lower amplitude (i.e. subconductive states) were not observed for either wt or TM channels, as demonstrated by current amplitude histograms shown in Fig. 2B.

Biophysical properties of RyR1 channel currents. (A) Current–voltage relationships of wt and T4709M single RyR1s. Average (± SE) wt and T4709M RyR1 channel slope-conductances are indicated in the right corner. (B) Representative amplitude histograms at -60 mV of wt and T4709M RyR1s.
As Ca2+ is one of the most important cytoplasmic ligands of RyRs, we compared the Ca2+ sensitivity of wt and TM channels. Based on this feature, TM channels clearly divided into two distinct populations. When the [Ca2+] on the cytoplasmic face of the channel was reduced from 50 μM, the open probability (Po) decreased in only 8 out of 16 TM channels (Fig. 3A), while the high PO of the other 8 TM channels was not reduced after lowering cytoplasmic [Ca2+], which is an extraordinarily high proportion of channels. In contrast, the high PO value of 12 out of 13 wt channels were reduced following reduction of cytoplasmic Ca2+, as is typical in our hands (i.e. ~ 10% Ca2+ unresponsive channels) (Fig. 3A). Representative current recordings from Ca2+ insensitive (or “reluctant”) and Ca2+ sensitive TM RyR1 channels, as well as a typical Ca2+ sensitive wt RyR1 in the presence of various cytoplasmic [Ca2+]s are shown in Fig. 3B. Comparing the Ca2+ sensitive TM population to wt revealed that the maximal channel activity measured at 50 μM Ca2+ was not significantly different between the two groups (Po = 0.55 ± 0.09 vs. 0.51 ± 0.12). We compared the Ca2+ dependence of the Ca2+ sensitive channels in 5; 1; 0.5; and 0.1 μM cytoplasmic [Ca2+] (Fig. 3B) and found that the Po of Ca2+ sensitive TM channels at 0.5 and 1 μM Ca2+ were significantly reduced compared to that of wt channels (p = 0.047 and 0.016), but Po values were not significantly different at 100 nM (resting) Ca2+ (p = 0.8) (Fig. 3C). These data agree with earlier data obtained in Q1970fsX16 + A4329D myopathy-mutant channels 34. The average Po of reluctant channels at 50 μM Ca2+ was substantially higher (0.89 ± 0.05) than that of their Ca2+ sensitive counterparts (0.55 ± 0.09), whereas their average Po did not decrease appreciably when the [Ca2+] was reduced to 500 nM (0.84 ± 0.11). It should be noted that we have not previously observed a similarly high proportion of reluctant channels in prior RyR1 preparations. This unusually high proportion of Ca2+-insensitive, high PO mutant channels would be expected to exhibit a “leaky” phenotype in spite of the absence of a significant proportion of subconduction states as is typically observed following FKBP dissociation. The reasons for the loss of Ca2+ regulation in this subpopulation of TM RyR1 channels is unclear, but could reflect either primary (conformational) and/or secondary (posttranslational) consequences of the mutation.

Ca2+ dependence of wt and T4709M RyR1 channel activity. (A) Pie charts summarizing the proportion of wt (left) and T4709M (right) RyR channels that responded to changes in cytosolic [Ca2+] (black). (B) Representative single channel current traces of wt and T4709M RyR1 channels at high (50 μM) and low (100 nM) cytosolic [Ca2+]. The closed state of the channel is marked by `c`. Downward deflections correspond to channel openings. Average open probabilities (Po) ± SE are indicated above each trace. (C) Relative RyR1 channel open probabilities (Po) (mean ± SE) plotted as a function of cytosolic [Ca2+]. Pos are expressed relative to control, recorded at 50 μM Ca2+.
Kushnir et al. reported that the TM mutation is located adjacent to the caffeine binding site and they used H3 Ryanodine-binding and single channel bilayer recordings of recombinant TM channels expressed in HEK cells to conclude that TM channels exhibit a normal low Po in low Ca2+ concentrations but an increased sensitivity to activation by caffeine (and thus, likely increased Ca2+ sensitivity)26, 44. Our results on TM/TM channels isolated from native skeletal muscle tissue are quite different or at least our observation of two different classes of channels (one with lower Ca2+ sensitivity and one with high Po at all Ca2+ concentrations) is different. This suggests that it is important to assess the function of RyR1 disease mutant channels purified from native skeletal muscle as their function can be influenced by numerous muscle-specific factors (e.g. post-translational modifications, binding proteins, luminal factors, etc.).
The Mg2+ and ATP sensitivity of wt and TM RyR1s were also tested. In the presence of 50 µM cytoplasmic [Ca2+], Mg2+ was added to the cytosolic face of the channels at increasing concentrations. The concentration-dependent inhibition of RyR1 activity (PO) by cytoplasmic Mg2+ not statistically different between wt and TM channels (Fig. 4A). Similarly, cytoplasmic ATP similarly activated both wt and TM RyR1 channels in a similar concentration dependent manner in the presence of 100 nM cytoplasmic Ca2+ in the absence of Mg2+ (Fig. 4B).

The effect of Mg2+– and ATP on the activity of wt and T4709M RyR1 channels. (A) Mean ± SE of Po of wt and T4709M RyR1 channels (50 μM cytosolic Ca2+) at different cytosolic Mg2+ concentrations normalized to control Po obtained at 0 μM Mg2+. (B) Mean ± SE of Po of wt and T4709M RyR1 channels (0 mM cytosolic Mg2+ and 100 nM cytosolic Ca2+) at different ATP concentrations normalized to control Po obtained at 0 μM ATP.
As the T4706M mutation is associated with increased susceptibility for malignant hyperthermia susceptibility41, we tested whether TM channels are sensitive to inhibition by dantrolene, which is the only drug used to treat a malignant hyperthermia crisis. Because dantrolene requires Mg2+ and ATP to inhibit the RyR1 channels in bilayers, we tested the effect of dantrolene in the presence of 1 mM ATP and 3 mM Mg2+ in the cytoplasmic compartment of the recording chamber48, 49. We found that a therapeutically relevant concentration of dantrolene (10 µM) produced a similar, significant (~ 60%) reduction in channel activity (PO) for both wt and TM channels (Fig. 5), demonstrating that TM channels retain dantrolene-sensitivity. It should be noted that while the Ca2+ sensitivity of the RyR1 channels evaluated in this series of experiments was not directly tested, based on the high Po value of the TM channels in 50 µM Ca2+ (0.93 ± 0.03), these RyR1 channels most likely represented Ca2+ insensitive “reluctant” channels.

The effect of dantrolene on wt and T4709M RyR1 channels. Control single channel currents of wt and T4709M RyR1 channels were recorded in the presence of 50 μM Ca2+, 1 mM ATP and 3 mM Mg2+. The channels were then treated with 10 μM dantrolene. Data are expressed as [(Po dantrolene/Po control) − 1] × 100.
Conclusion
In this study, we identified two functionally distinct populations of T4706M RyRs. One group exhibited high baseline Po that was not reduced following a reduction in cytoplasmic Ca2+ to physiological levels observed in resting skeletal muscle (e.g. 100 nM), which would be expected to result in a high level of RyR1-dependent SR Ca2+ leak. While the other population of TM RyR1 channels exhibited a normal PO at 100 nM cytoplasmic Ca2+, these channels showed lower responsiveness to moderate elevations in cytoplasmic Ca2+ (0.5–10 μM) that occur during muscle excitation. Our results suggest that Ca2+-insensitive, high PO (i.e. reluctant) channels will enhance resting SR Ca2+ leak and potentially deplete SR Ca2+, which would reduce the driving force for Ca2+-release and contribute to T4706M-linked myopathy. In addition, the reduced Ca2+-sensitivity a subpopulation of TM RyR1 channels may also participate in the pathomechanism of muscle weakness by since these channels would be less activated by cytoplasmic Ca2+ during muscle excitation. We must note that functional dichotomy of homotypic TM channels may reflect either primary conformational or secondary post-translational (e.g. Ca2+-dependent modifications) mechanisms. Although, our description of two functionally distinct classes of RyR1 channels in TM muscle falls short of determining their relative role in disease pathogenesis, these results should be taken into consideration in the development of new therapeutic interventions for RyR1-related myopathy.
Data availability
All data obtained or analyzed during this study are included in this published article.
References
-
Fill, M. & Copello, J. A. Ryanodine receptor calcium release channels. Physiol. Rev. 82, 893–922 (2002).
Google Scholar
-
Calderón, J. C., Bolaños, P. & Caputo, C. The excitation–contraction coupling mechanism in skeletal muscle. Biophys. Rev. 6, 133–160 (2014).
Google Scholar
-
Smith, J. S., Coronado, R. & Meissner, G. Sarcoplasmic reticulum contains adenine nucleotide-activated calcium channels. Nature 316, 446–449 (1985).
Google Scholar
-
Smith, J. S., Coronado, R. & Meissner, G. Single channel measurements of the calcium release channel from skeletal muscle sarcoplasmic reticulum. Activation by Ca2+ and ATP and modulation by Mg2+. J. Gen. Physiol. 88, 573–588 (1986).
Google Scholar
-
Smith, J. S. et al. Purified ryanodine receptor from rabbit skeletal muscle is the calcium-release channel of sarcoplasmic reticulum. J. Gen. Physiol. 92, 1–26 (1988).
Google Scholar
-
Sárközi, S. et al. Regulation of the rat sarcoplasmic reticulum calcium release channel by calcium. J. Muscle Res. Cell Motil. 21, 131–138 (2000).
Google Scholar
-
Jóna, I. et al. Altered inhibition of the rat skeletal ryanodine receptor/calcium release channel by magnesium in the presence of ATP. Pflügers Arch. Eur. J. Physiol. 441, 729–738 (2001).
-
Laver, D. R. Regulation of the RyR channel gating by Ca2+ and Mg2. Biophys. Rev. 10, 1087–1095 (2018).
Google Scholar
-
Laver, D. R., Lenz, G. K. E. & Lamb, G. D. Regulation of the calcium release channel from rabbit skeletal muscle by the nucleotides ATP, AMP, IMP and adenosine. J. Physiol. 537, 763–778 (2001).
Google Scholar
-
Ebashi, S. & Endo, M. Calcium and muscle contraction. Prog. Biophys. Mol. Biol. 18, 123–183 (1968).
Google Scholar
-
Endo, M. Calcium-induced calcium release in skeletal muscle. Physiol. Rev. 89, 1153–1176 (2009).
Google Scholar
-
Belevych, A. E., Radwański, P. B., Carnes, C. A. & Györke, S. ‘Ryanopathy’: Causes and manifestations of RyR2 dysfunction in heart failure. Cardiovasc. Res. 98, 240–247 (2013).
Google Scholar
-
Lawal, T. A., Todd, J. J. & Meilleur, K. G. Ryanodine receptor 1-related myopathies: Diagnostic and therapeutic approaches. Neurotherapeutics 15, 885–899 (2018).
Google Scholar
-
Dowling JJ, N. K. G. H. B. A. (2015) Neuromuscular Disorders of Infancy, Childhood, and Adolescence: A clinician’s approach, 2nd Ed. (Darras BT, Jones HR, Ryan MM, and Vivo DCD eds), Elsevier, Academic Press, San Diego
-
Riazi, S., Kraeva, N. & Hopkins, P. M. Malignant hyperthermia in the post-genomics era: New perspectives on an old concept. Anesthesiology 128, 168–180 (2018).
Google Scholar
-
Kolb, M. E., Horne, M. L. & Martz, R. Dantrolene in human malignant hyperthermia a multicenter study. Anesthesiology 56, 254–262 (1982).
Google Scholar
-
MacLennan, D. H. & Phillips, M. S. Malignant hyperthermia. Science 256, 789–794 (1992).
Google Scholar
-
Flewellen, E. H., Nelson, T. E., Jones, W. P., Arens, J. F. & Wagner, D. L. Dantrolene dose response in awake man: Implications for management of malignant hyperthermia. Anesthesiology 59, 275–280 (1983).
Google Scholar
-
Van Winkle, W. B. Calcium release from skeletal muscle sarcoplasmic reticulum: Site of action of dantrolene sodium?. Science 193, 1130–1131 (1976).
Google Scholar
-
Fruen, B. R., Mickelson, J. R. & Louis, C. F. Dantrolene inhibition of sarcoplasmic reticulum Ca2+ release by direct and specific action at skeletal muscle ryanodine receptors. J. Biol. Chem. 272, 26965–26971 (1997).
Google Scholar
-
Zhou, H. et al. Characterization of recessive RYR1 mutations in core myopathies. Hum. Mol. Genet. 15, 2791–2803 (2006).
Google Scholar
-
Zhou, H. et al. RyR1 deficiency in congenital myopathies disrupts excitation-contraction coupling. Hum. Mutat. 34, 986–996 (2013).
Google Scholar
-
Avila, G. & Dirksen, R. T. Functional effects of central core disease mutations in the cytoplasmic region of the skeletal muscle ryanodine receptor. J. Gen. Physiol. 118, 277–290 (2001).
Google Scholar
-
Treves, S., Jungbluth, H., Muntoni, F. & Zorzato, F. Congenital muscle disorders with cores: The ryanodine receptor calcium channel paradigm. Curr. Opin. Pharmacol. 8, 319–326 (2008).
Google Scholar
-
Ebersole, J. S. & Levine, R. A. Abnormal neuronal responses during evolution of a penicillin epileptic focus in cat visual cortex. J. Neurophysiol. 38, 250–256 (1975).
Google Scholar
-
Kushnir, A. et al. Intracellular calcium leak as a therapeutic target for RYR1-related myopathies. Acta Neuropathol. 139, 1089–1104 (2020).
Google Scholar
-
Reiken, S. et al. PKA phosphorylation activates the calcium release channel (ryanodine receptor) in skeletal muscle. J. Cell Biol. 160, 919–928 (2003).
Google Scholar
-
Aracena, P., Tang, W., Hamilton, S. L. & Hidalgo, C. Effects of S -glutathionylation and S -nitrosylation on calmodulin binding to triads and FKBP12 binding to type 1 calcium release channels. Antioxid. Redox Signal. 7, 870–881 (2005).
Google Scholar
-
Bellinger, A. M. et al. Hypernitrosylated ryanodine receptor calcium release channels are leaky in dystrophic muscle. Nat. Med. 15, 325–330 (2009).
Google Scholar
-
Bellinger, A. M. et al. Remodeling of ryanodine receptor complex causes “leaky” channels: A molecular mechanism for decreased exercise capacity. Proc. Natl. Acad. Sci. 105, 2198–2202 (2008).
Google Scholar
-
Andersson, D. C. et al. Ryanodine receptor oxidation causes intracellular calcium leak and muscle weakness in aging. Cell Metab. 14, 196–207 (2011).
Google Scholar
-
Rullman, E. et al. Modifications of skeletal muscle ryanodine receptor type 1 and exercise intolerance in heart failure. J. Hear. Lung Transplant. 32, 925–929 (2013).
-
Dowling, J. J. et al. Oxidative stress and successful antioxidant treatment in models of RYR1-related myopathy. Brain 135, 1115–1127 (2012).
Google Scholar
-
Elbaz, M. et al. Quantitative RyR1 reduction and loss of calcium sensitivity of RyR1Q1970fsX16+A4329D cause cores and loss of muscle strength. Hum. Mol. Genet. 28, 2987–2999 (2019).
Google Scholar
-
Yuan, Q. et al. RyR1-related myopathy mutations in ATP and calcium binding sites impair channel regulation. Acta Neuropathol. Commun. 9, 186 (2021).
Google Scholar
-
Neto, O. A. et al. Common and variable clinical, histological, and imaging findings of recessive RYR1-related centronuclear myopathy patients. Neuromuscul. Disord. 27, 975–985 (2017).
-
Gonzalez-Quereda, L. et al. Targeted next-generation sequencing in a large cohort of genetically undiagnosed patients with neuromuscular disorders in spain. Genes (Basel) 11, 539 (2020).
Google Scholar
-
Foo, C. T. Y. et al. Variant landscape of the RYR1 gene based on whole genome sequencing of the Singaporean population. Sci. Rep. 12, 5429 (2022).
Google Scholar
-
Bharucha-Goebel, D. X. et al. Severe congenital RYR1-associated myopathy: The expanding clinicopathologic and genetic spectrum. Neurology 80, 1584–1589 (2013).
Google Scholar
-
Gonorazky, H. D., Bönnemann, C. G. & Dowling, J. J. The genetics of congenital myopathies. Handb. Clin. Neurol. 148, 549–564. https://doi.org/10.1016/B978-0-444-64076-5.00036-3 (2018).
Google Scholar
-
Colombo, I. et al. Congenital myopathies: Natural history of a large pediatric cohort. Neurology 84, 28–35 (2015).
Google Scholar
-
Brennan, S. et al. Mouse model of severe recessive RYR1-related myopathy. Hum. Mol. Genet. 28, 3024–3036 (2019).
Google Scholar
-
Zhou, H. et al. Epigenetic allele silencing unveils recessive RYR1 mutations in core myopathies. Am. J. Hum. Genet. 79, 859–868 (2006).
Google Scholar
-
des Georges, A. et al. Structural basis for gating and activation of RyR1. Cell 167, 145-157.e17 (2016).
Google Scholar
-
Sárközi, S., Komáromi, I., Jóna, I. & Almássy, J. Lanthanides report calcium sensor in the vestibule of ryanodine receptor. Biophys. J. 112, 2127–2137 (2017).
Google Scholar
-
Szigeti, G. P. et al. Alterations in the calcium homeostasis of skeletal muscle from postmyocardial infarcted rats. Pflügers Arch. Eur. J. Physiol. 455, 541–553 (2007).
Google Scholar
-
Geyer, N., Diszházi, G., Csernoch, L., Jóna, I. & Almássy, J. Bile acids activate ryanodine receptors in pancreatic acinar cells via a direct allosteric mechanism. Cell Calcium 58, 160–170 (2015).
Google Scholar
-
Diszházi, G. et al. Dantrolene requires Mg2+ and ATP to inhibit the ryanodine receptor. Mol. Pharmacol. 96, 401–407 (2019).
Google Scholar
-
Choi, R. H., Koenig, X. & Launikonis, B. S. Dantrolene requires Mg 2+ to arrest malignant hyperthermia. Proc. Natl. Acad. Sci. 114, 4811–4815 (2017).
Google Scholar
Acknowledgements
This work was funded by the RyR1 Foundation (Pittsburg, PA, USA, https://ryr1.org/), the National Research Development and Innovation Fund of Hungary provided to J.A. (NKFIH FK144576), and the National Institutes for Health (AR078000 to R.T.D.). J.A. is supported by the Janos Bolyai Research Scholarship of the Hungarian Academy of Sciences. Z.É.M.’s work was supported by the New National Excellence Program (ÚNKP-22-4-I-DE-28) of the Ministry for Innovation and Technology of Hungary.
Funding
Open access funding provided by Semmelweis University.
Author information
Authors and Affiliations
Contributions
Z.É.M. and J.H. performed experiments, analyzed data, visualized the results, wrote the original draft. L.G. performed experiments. R.T.D. supervised the study, edited the manuscript. J.A. designed the experiments, supervised the study, interpreted data, wrote the original draft, acquired funding. All authors revised and approved the submitted version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
Reprints and Permissions
About this article
Cite this article
Magyar, Z.É., Hevesi, J., Groom, L. et al. Function of a mutant ryanodine receptor (T4709M) linked to congenital myopathy.
Sci Rep 13, 14659 (2023). https://doi.org/10.1038/s41598-023-41801-2
-
Received: 29 January 2023
-
Accepted: 31 August 2023
-
Published: 05 September 2023
-
DOI: https://doi.org/10.1038/s41598-023-41801-2
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.