Cardiovascular
Food additive emulsifiers and risk of cardiovascular disease in the NutriNet-Santé cohort: prospective cohort study
Abstract
Objective To assess the associations between exposure to food additive emulsifiers and risk of cardiovascular disease (CVD).
Design Prospective cohort study.
Setting French NutriNet-Santé study, 2009-21.
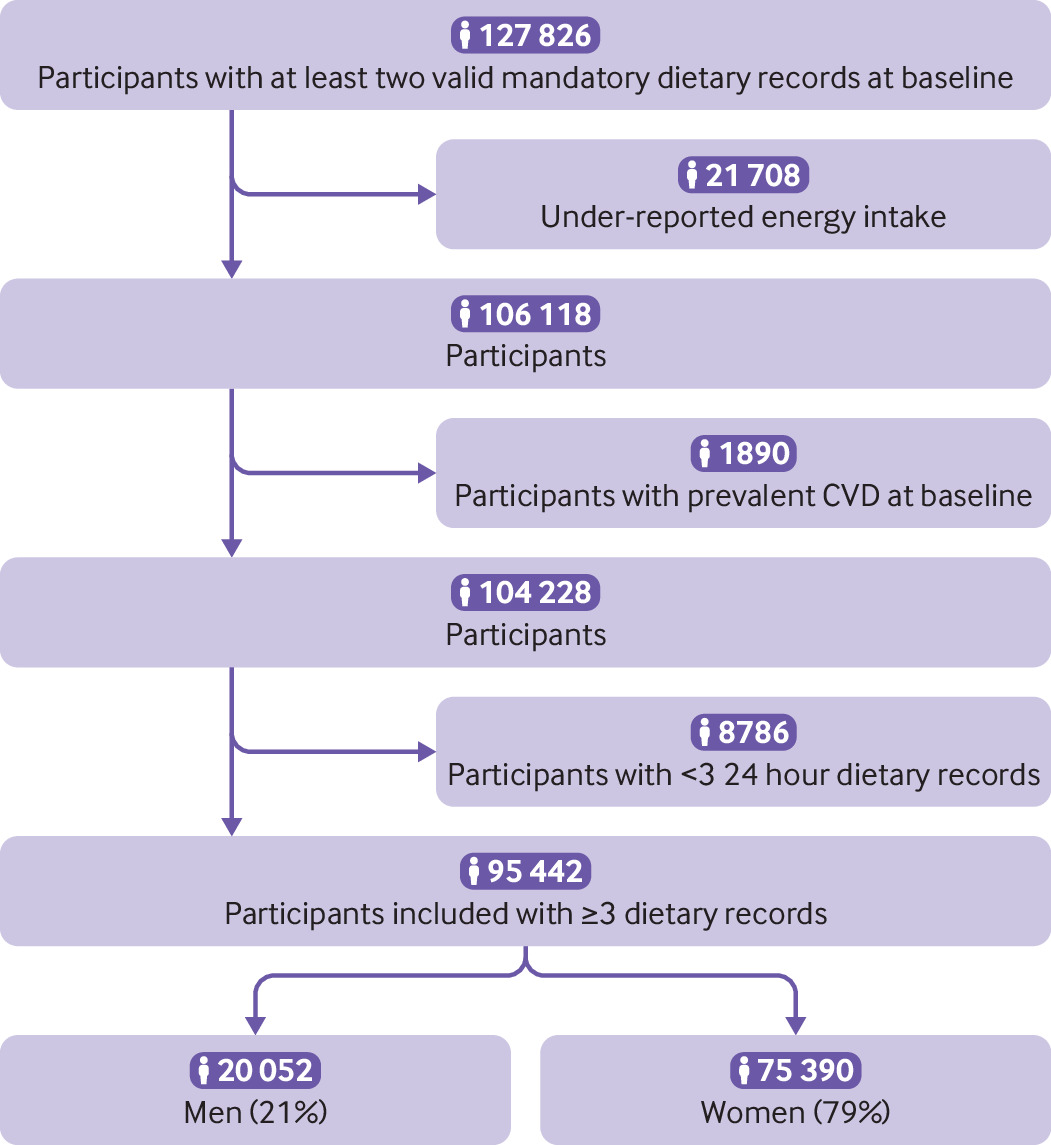
Participants 95 442 adults (>18 years) without prevalent CVD who completed at least three 24 hour dietary records during the first two years of follow-up.
Main outcome measures Associations between intake of food additive emulsifiers (continuous (mg/day)) and risk of CVD, coronary heart disease, and cerebrovascular disease characterised using multivariable proportional hazard Cox models to compute hazard ratios for each additional standard deviation (SD) of emulsifier intake, along with 95% confidence intervals.
Results Mean age was 43.1 (SD 14.5) years, and 79.0% (n=75 390) of participants were women. During follow-up (median 7.4 years), 1995 incident CVD, 1044 coronary heart disease, and 974 cerebrovascular disease events were diagnosed. Higher intake of celluloses (E460-E468) was found to be positively associated with higher risks of CVD (hazard ratio for an increase of 1 standard deviation 1.05, 95% confidence interval 1.02 to 1.09, P=0.003) and coronary heart disease (1.07, 1.02 to 1.12, P=0.004). Specifically, higher cellulose E460 intake was linked to higher risks of CVD (1.05, 1.01 to 1.09, P=0.007) and coronary heart disease (1.07, 1.02 to 1.12, P=0.005), and higher intake of carboxymethylcellulose (E466) was associated with higher risks of CVD (1.03, 1.01 to 1.05, P=0.004) and coronary heart disease (1.04, 1.02 to 1.06, P=0.001). Additionally, higher intakes of monoglycerides and diglycerides of fatty acids (E471 and E472) were associated with higher risks of all outcomes. Among these emulsifiers, lactic ester of monoglycerides and diglycerides of fatty acids (E472b) was associated with higher risks of CVD (1.06, 1.02 to 1.10, P=0.002) and cerebrovascular disease (1.11, 1.06 to 1.16, P<0.001), and citric acid ester of monoglycerides and diglycerides of fatty acids (E472c) was associated with higher risks of CVD (1.04, 1.02 to 1.07, P=0.004) and coronary heart disease (1.06, 1.03 to 1.09, P<0.001). High intake of trisodium phosphate (E339) was associated with an increased risk of coronary heart disease (1.06, 1.00 to 1.12, P=0.03). Sensitivity analyses showed consistent associations.
Conclusion This study found positive associations between risk of CVD and intake of five individual and two groups of food additive emulsifiers widely used in industrial foods.
Introduction
In Europe and North America, 30-60% of dietary energy intake in adults is provided by ultra-processed foods—highly processed products often formulated using cosmetic food additives and ingredients of rare culinary use, which have resulted in considerable research interest in the past few years.123 Recent epidemiological studies have linked high intakes of ultra-processed foods with higher risks of obesity and mortality and non-communicable diseases, such as cancers, cardiovascular diseases (CVD), and type 2 diabetes.4 One major hypothesis proposed to explain these associations is the potential deleterious properties of some food additives, which are used ubiquitously in ultra-processed foods.45
In particular, emulsifiers are among the most commonly used additives in industrial foods owing to their emulsifying and thickening properties that improve texture and lengthen shelf-life.6 Although no worldwide estimate of emulsifier use in the food industry exists, a recent descriptive study of the NutriNet-Santé prospective cohort study revealed that seven of the 10 most consumed food additives among French adults were classified as emulsifiers (total modified starches, lecithins, xanthan gum, pectins, monoglycerides and diglycerides of fatty acids, carrageenan, and guar gum), and modified starches were consumed by more than 90% of the participants.7 Additionally, more than 53.8% of food or beverage industrial products contain at least one food emulsifier5 as estimated from Open Food Facts,8 a database that contains information and data on food products from around the world.
Despite their evaluation of safety and acceptable daily intakes provided by the European Food Safety Authority, recent experimental studies suggested potential deleterious effects of food additive emulsifiers on the gut microbiota and gut inflammation.9101112131415 In particular, a recent randomised controlled trial in healthy individuals found that compared with an equivalent additive-free diet, short term intake of 15 g/day (supraphysiological doses) of carboxymethylcellulose (European code E466) increased postprandial abdominal discomfort and rapidly altered the composition and localisation of intestinal microbiota as well as the production of intestinal metabolites,16 the last of these having shown associations with CVD.17
The large epidemiological NutriNet-Santé cohort study collected detailed information on specific commercial brands of industrial food consumed, and performed an estimation of quantitative exposures to food additives individually (including emulsifiers) among more than 100 000 French adults.7 This work provides the basis for aetiological studies, which are crucially needed to generate hypotheses on the role of food additives on long term health outcomes. The present study assessed the association between intakes of food additive emulsifiers (total and specific substances) and CVD risk among French adults from the NutriNet-Santé prospective cohort study.
Methods
Study population
This study was based on the prospective NutriNet-Santé e-cohort, launched in May 2009, with an open ongoing enrolment of volunteers and the main objective of investigating the associations between nutrition and health.18 Participants are recruited from the general population of French adults (>18 years) through multimedia campaigns. To enrol, participants are required to create a personal account on the NutriNet-Santé web-based platform (https://etude-nutrinet-sante.fr/). Upon enrolment, participants are invited to complete five questionnaires about their dietary intakes, health (eg, personal and family history of disease, prescribed drugs), anthropometric data (eg, height, weight),1920 physical activity (validated seven day assessment through the International Physical Activity Questionnaire),21 lifestyle and sociodemographic data (eg, date of birth, sex, education level, professional occupation, smoking status, number of children).22
Dietary data collection
Usual dietary intakes were assessed at inclusion and then every six months, using repeated sets of three non-consecutive web-based 24 hour dietary records, randomly assigned over a two week period (two weekdays and one weekend day). The NutriNet-Santé web-based self-administered 24 hour dietary records have shown good performances when tested against an interview with a trained dietitian23 and against blood and urinary biomarkers (showing appropriate estimates of true intakes of fruit, vegetables, fish, β carotene, vitamin C, omega 3 fatty acids, proteins, and potassium).2425 In this analysis, we calculated the usual baseline dietary intakes as the average of all 24 hour dietary records completed during the first two years of each participant’s follow-up, with a mandatory requirement of having at least completed three valid days of 24 hour dietary records during this period to be included in the analysis.
At all times throughout their assigned dietary record period, participants had access to a dedicated interface of the study website to report all foods and beverages consumed during a 24 hour period: three main meals (breakfast, lunch, dinner) and any other eating occasion. The dietary assessments included details of commercial names and brands of industrial products, to determine individual additive intake. Participants were asked to estimate portion sizes either by entering the weight or volume of food consumed directly in the platform, or by using validated photographs or usual containers.26 A French food composition database (>3500 items)27 was used to estimate mean daily intakes of energy, alcohol, macronutrients, and micronutrients. These estimates included contributions from composite dishes using French recipes validated by food and nutrition professionals. Respondents who under-reported total energy intake were identified and excluded based on the method proposed by Black28 from the original method developed by Goldberg.29 Several quality control operations were also performed to account for over-reporting (see supplementary eMethod1).
Emulsifier intakes
We quantified the intakes of food additives on the basis of data provided in the participants’ dietary records, in which the commercial brand or name of the industrial products consumed were recorded. The method for quantification of food additive intakes has been described previously.7 Briefly, for qualitative assessment we matched each food item consumed and reported in a specific dietary record against three databases to identify the presence of any food additive: OQALI,30 a national database managed by the Institut National de la Recherche pour l’Agriculture, l’alimentation et l’Environnement and the French food safety authority (Agence Nationale de Sécurité Sanitaire de l’Alimentation, de l’Environnement et du Travail) to characterise the quality of the food supply, Open Food Facts, an open collaborative database of food products marketed worldwide,8 and the Mintel Global New Products Database,31 an online database of innovative food products worldwide. For quantitative assessment, we estimated the dose of food additive ingested with each food item, following a decision tree with a descending order of detail level, based on ad hoc laboratory assays quantifying additives in specific food items (n=2677 food-additive pairs analysed targeted among the main vectors of these additives in our study population, and performed by Mérieux and Eurofins firms and the French Directorate General for Competition Policy, Consumer Affairs and Fraud Control public laboratories), doses in generic food categories provided by the European Food Safety Authority, or generic doses from the Codex General Standard for Food Additives32 (see supplementary eMethod2 for details). We applied dynamic matching—that is, products were matched date-to-date, whereby the date of consumption of each food or beverage declared by each participant was used to match the product to the closest composition data, thus accounting for potential reformulations.
Among the available food additives quantified from the participants’ dietary records, we identified 61 food additives classified as emulsifiers or emulsifying salts from the 261 additives under the functional class “emulsifier” or “emulsifying salt” of Codex General Standard for Food Additives database,32 or according to US or UK regulations when not included in Codex (eg, E404, E418, E468)6 and considered the sum of intakes as intake of total emulsifiers (see table 2). In addition, we summed individual emulsifiers with similar chemical structures into eight groups: total phosphates (E339, E340, E341, E343, E450, E451, E452), total lactylates (E481, E482), total polyglycerol esters of fatty acids (E475, E476), total monoglycerides and diglycerides of fatty acids (E471, E472, E472a-b-c-e), total celluloses (E460, E461, E464, E466, E468), total carrageenans (E407, E407a), total alginates (E400, E401, E402, E404, E405), and total modified starches (generic European Union code for this category E14xx).
CVD ascertainment
Participants were invited to declare any major health event, either through the yearly health status questionnaire (a specific health check-up questionnaire sent out every six months) or spontaneously at any time on a dedicated interface on the study website. We asked participants to send their medical records (eg, complementary examinations for diagnosis, hospital admissions, or anatomopathological reports) to support any declaration of a health event. A physician expert committee validated each major health event after reviewing the participants’ medical records and collecting additional information from the participants’ doctors or medical facilities. In the absence of any response to the study website for more than one year, the physician expert committee contacted the participants’ family or physicians. In addition to this process, which constituted the main source of event ascertainment, we linked cohort data from participants to medico-administrative databases from the National Health Insurance (authorisation by the Council of State No 2013-175). Finally, the French national cause specific mortality registry (CépiDC) was used to identify mortality linked to CVD events and to other causes of death that were considered as competing events.
Participants with CVD were identified using ICD-10-CM (international classification of diseases-clinical modification, 10th revision) codes. In this study, we considered as events all primary CVD diagnosed between the inclusion date and 5 October 2021, which included coronary heart diseases such as myocardial infarction (code I21), acute coronary syndrome (I21.4), angioplasty (Z95.8), and angina pectoris (I20.0), along with cerebrovascular diseases such as stroke (I64) and transient ischaemic attack (G45.8 and G45.9).
Statistical analyses
For this study, we included participants from the NutriNet-Santé cohort who completed at least three 24 hour dietary records during their first two years of follow-up and had no diagnosis of any prevalent CVD at baseline. Characteristics of the study sample according to sex specific fourths of intake of total emulsifiers were compared using Kruskal-Wallis rank sum test or Pearson’s χ2 test. In addition, we generated a correlation matrix to visualise the relationships between intakes of individual emulsifiers (see supplementary eFigure1).
We assessed the associations between intakes of emulsifiers (continuous) and risks of CVD, coronary heart disease, and cerebrovascular disease using multivariable proportional hazard Cox models, which computed hazard ratios per additional standard deviation ((SD) to provide a standardised increment given the difference in the distribution and the amounts of the different emulsifiers) of intake and 95% confidence intervals. To ensure acceptable statistical power, we restricted analyses on individual emulsifiers to those consumed by at least 5% of the included participants. The proportional hazard assumption was tested using the Schoenfeld residual method implemented in the survival R package (see supplementary eFigure2),3334 and the log linearity between emulsifier intakes and hazard ratios was assessed using restricted cubic splines (see supplementary eFigure3).35 Participants contributed person time to the models until the date of CVD diagnosis, date of death, date of last completed questionnaire, or 5 October 2021, whichever occurred first. Cause specific hazard ratios were computed so that death and CVD events other than the one studied (for coronary heart disease and cerebrovascular disease specific analyses) occurring during follow-up were handled as competing risks. When values for covariates were missing, we used multiple imputation by additive regression, followed by bootstrapping, and predictive mean matching (n=20 imputed dataset) as implemented in the Hmisc R package (see supplementary eMethod3).36
The adjustment strategy was defined according to a directed acyclic graph (see supplementary eFigure4). The main model was adjusted for age (timescale); sex; body mass index (BMI, continuous); physical activity (categorical International Physical Activity Questionnaire variable: high, moderate, low); smoking status, (never smoker, former smoker, occasional smoker, regular smoker); number of smoked cigarettes in pack years (continuous); educational level (less than high school degree, <2 years after high school degree, ≥2 years after high school degree); family history of CVD (yes/no); daily alcohol intake (continuous, g/day); consumption of fruit and vegetables (continuous, g/day), red and processed meats (continuous, g/day), and wholegrain foods (continuous, g/day); proportion of ultra-processed food consumed in the diet, in weight (continuous, %), as defined by the NOVA classification37; and the number of dietary records (continuous). In addition, even if not considered as direct confounders by the directed acyclic graph, we further adjusted each model for intakes of energy without alcohol (continuous, kcal/day), saturated fatty acids (continuous, g/day), sodium (continuous, mg/day), total fibre (continuous, g/day), and total sugars (continuous, g/day), as markers of overall diet nutritional quality or for having strong links with CVD risk.
We conducted sensitivity analyses for all emulsifiers with at least one statistically significant association with risk of CVD, coronary heart disease, or cerebrovascular disease. The false discovery rate was used to adjust P values obtained from the main model for multiple testing.38 In sensitivity analyses, model 1 was further adjusted for healthy and western dietary patterns derived by principal component analyses (see supplementary eMethod4). Model 2 was based on the main model and further adjusted for the diagnosis or treatment, or both, of at least one prevalent metabolic disorder (ie, type 2 diabetes, hypertriglyceridaemia, hypertension). Model 3 was based on the main model and further adjusted for the percentage of weight change from baseline. Model 4 was based on the main model and excluded participants with CVD diagnosed during the first two years of follow-up. Model 5 was based on the main model, using the average of all available 24 hour dietary records throughout the follow-up of each participant (≤62 records per participant) instead of averaged values on their first two years of follow-up. Model 6 was based on the main model and further adjusted for the intakes of other emulsifiers than the one studied in the model, and for total artificial sweeteners.39 Finally, we conducted analyses for all emulsifiers with at least one statistically significant association with the specific outcomes of stroke, myocardial infarction, angioplasty, acute coronary syndrome, transient ischaemic attack, angina pectoris, and severe CVD (ie, total CVD except for transient ischaemic attack and angina pectoris). We found no evidence in the mechanistic literature on specific interactions (for example, with BMI and age), or sexual dimorphism, for the association between intake of emulsifiers and CVD risk.
All statistical tests were two sided, and we considered P<0.05 to be significant. All statistical analyses were conducted in R version 4.1.2,40 except for the restricted cubic spline method, which was implemented in SAS version 9.4.
Patient and public involvement
The research question developed in this article corresponds to a strong concern of the participants involved in the NutriNet-Santé cohort, and of the public in general. Even though the cohort was launched before patient and public involvement was common, the research our team carries out deals with timely societal public health nutrition topics. Investigators of the NutriNet-Santé cohort regularly deliver presentations to the lay public and patients and participate in media interviews, where they share the latest results, screen the public’s current interest in the specialty of nutrition and health, and encourage enrolment in the cohort.
Results
Descriptive characteristics
A total of 95 442 adults (>18 years) were included in the study (fig 1), most of whom were women (n=75 390, 79.0%). Table 1 lists the baseline characteristics of the participants. Mean age was 43.1 (SD 14.5) years, and the average number of dietary records was 6.0 (SD 3.0). Supplementary eFigure5 shows the distribution of the number of dietary records for each participant. At baseline, compared with participants with the lowest intakes, those with the highest intakes of emulsifiers were more likely to be younger, to have a higher BMI, to be never smokers, to have higher education and physical activity levels, and to have higher intakes of energy, saturated fats, sodium, sugars, and fibre, and lower intakes of alcohol. They consumed less fruit, vegetables, and whole grain foods and more red and processed meats and ultra-processed foods (table 1).
Flowchart of participants included from NutriNet-Santé cohort, 2009-21 (n=95 442)
“>
Flowchart of participants included from NutriNet-Santé cohort, 2009-21 (n=95 442)
Baseline characteristics of study participants from the NutriNet-Santé cohort according to sex specific fourths of intakes of total emulsifiers, 2009-21. Values are mean (standard deviation) unless stated otherwise
The main contributors to total emulsifier intake were modified starches (E14xx, 33.5%), sodium bicarbonate (E500, 26.9%), pectins (E440, 6.4%), diphosphates (E450, 5.1%), and monoglycerides and diglycerides of fatty acids (E471, 5.0%) (fig 2). Overall, correlations between intakes of individual emulsifiers were limited (see supplementary eFigure1). Table 2 shows detailed intakes of individual and groups of emulsifiers. A total of 32 individual emulsifiers were consumed by <5% of the included participants and were therefore not studied individually in relation to CVD risk: E400, E468, E444, E482, E491, E492, E402, E433, E472, E967, E445, E477, E418, E406, E965, E1505, E461, E473, E551, E472a, E464, E404, E405, E343, E332, E335, E425, E435, E541, E900, E999, and E1520 (table 2); however, they contributed to the sum of total emulsifiers. Food additive emulsifiers were mostly found in processed fruit and vegetables (eg, dehydrated soups) (contributing to 18.8% of total emulsifier intakes), cakes and biscuits (14.7%), and dairy products (9.9%) (fig 3, supplementary eTable1). The most important dietary sources of total celluloses were cakes and biscuits (43.4%) and processed potatoes and tubers (20.1%), whereas those of total monoglycerides and diglycerides of fatty acids were fats and sauces (eg, packaged mayonnaise) (22.5%) and cakes and biscuits (22.0%).
“>
Contribution of individual emulsifiers to total emulsifier intakes (%) among participants from the NutriNet-Santé cohort, 2009-21 (n=95 442). Other emulsifiers included triphosphates (European code E451), gum arabic (E414), polyphosphates (E452), carob bean gum (E410), cellulose (E460), tricalcium phosphate (E341), monoacetyl and diacetyl tartaric acid esters of monoglycerides and diglycerides of FAs (E472e), hydroxypropyl methylcellulose (E464), polyglycerol esters of FAs (E475), lactic acid esters of monoglycerides and diglycerides of FAs (E472b), polydextrose (E1200), sodium stearoyl-2-lactylate (E481), sodium alginate (E401), ammonium salts of phosphatidic acid (E442), esters of monoglycerides and diglycerides of FAs (E472), polyglycerol esters of interesterified ricinoleic acid (E476), citric acid esters of monoglycerides and diglycerides of FAs (E472c), silicon dioxide (E551), tripotassium phosphate (E340), methylcellulose (E461), carboxymethylcellulose (E466), trisodium phosphate (E339), acetic acid esters of monoglycerides and diglycerides of FAs (E472a), agar (E406), sucrose esters of FAs (E473), propylene glycol esters of FAs (E477), gellan gum (E418), sorbitan tristearate (E492), processed Euchema seaweed (E407a), beeswax (E901), potassium alginate (E402), maltitol (E965), triethyl citrate (E1505), xylitol (E967), glycerol esters of rosin (E445), polyoxyethylene sorbitan monooleate (E433), potassium dihydrogen citrate (E332), calcium alginate (E404), calcium stearoyl-2-lactylate (E482), konjac flour (E425), cross linked sodium carboxymethylcellulose (E468), sucrose acetate isobutyrate (E444), sodium tartarate (E335), polyoxyethylene sorbitan monostearate (E435), sorbitan monostearate (E491), alginic acid (E400), propylene glycol (E1520), Quillaia extract (E999), sodium aluminium phosphate (E541), magnesium hydrogen phosphate (E343), propylene glycol alginate (E405), and dimethyl polysiloxane (E900). FAs=fatty acids
Daily emulsifier intakes among study participants from the NutriNet-Santé cohort, 2009-21 (n=95 442)
“>
Dietary sources of total and groups of emulsifier intakes among study participants from the NutriNet-Santé cohort, 2009-21 (n=95 442). Groups of emulsifiers were defined as (European codes) total phosphates (E339, E340, E341, E343, E450, E451, E452), total lactylates (E481, E482), total polyglycerol esters of FAs (E475, E476), total monoglycerides and diglycerides of FAs (E471, E472, E472a, E472b, E472c, E472e), total celluloses (E460, E461, E464, E466, E468), total carrageenans (E407, E407a), total alginates (E400, E401, E402, E404, E405), and total modified starches (E14xx). Also see supplementary eTable1. FAs=fatty acids; NA=not applicable
Associations between emulsifier intakes and CVD risk
After a mean follow-up of 7.0 (median 7.4, interquartile range 3.5-10.2) years, 1995 incident CVD events were diagnosed between 2009 and 2021 (666 509 person years), including 1044 coronary heart disease and 974 cerebrovascular disease events. Schoenfeld residuals did not show evidence for violation of the proportional hazard assumptions (see supplementary eFigure2), and restricted cubic spline plots (see supplementary eFigure3) globally supported the linearity of the observed associations.
Figure 4 shows the main associations between emulsifier intakes and CVD risk (emulsifiers shown if at least one association was statistically significant with one of the studied outcomes) and supplementary eTable2 provides all associations in detail. Higher intake of total celluloses (E460-E468) was associated with higher risks of CVD (hazard ratio for an increase of 1 standard deviation 1.05, 95% confidence interval 1.02 to 1.09, P=0.004) and coronary heart disease (1.07, 1.02 to 1.12, P=0.004). Specifically, higher cellulose (E460) intake was associated with higher risk of CVD (1.05, 1.01 to 1.09, P=0.007) and coronary heart disease (1.07, 1.02 to 1.12, P=0.005); and higher intake of carboxymethylcellulose (E466) was associated with higher risks of CVD (1.03, 1.01 to 1.05, P=0.004) and coronary heart disease (1.04, 1.02 to 1.06, P=0.001). Additionally, higher intakes of total monoglycerides and diglycerides of fatty acids (E471 and E472) were associated with higher risks of all three outcomes: CVD (1.07, 1.04 to 1.11, P<0.001), coronary heart disease (1.08, 1.03 to 1.14), P=0.001), and cerebrovascular disease (1.07, 1.01 to 1.13, P=0.02). Within this group of emulsifiers, lactic ester of monoglycerides and diglycerides of fatty acids (E472b) was associated with higher risk of CVD (1.06, 1.02 to 1.10, P=0.004) and cerebrovascular disease (1.11, 1.06 to 1.16, P<0.001). Citric acid ester of monoglycerides and diglycerides of fatty acids (E472c) was associated with higher risk of CVD (1.04, 1.02 to 1.07, P=0.004) and coronary heart disease (1.06, 1.03 to 1.09, P0.5, see supplementary eTable2).
“>
Associations between selected emulsifier intakes and risk of CVD among participants from the NutriNet-Santé cohort, 2009-21 (n=95 442). Hazard ratio for an increment of 1 standard deviation. Groups of emulsifiers were defined as (European codes) total monoglycerides and diglycerides of FAs (E471, E472, E472a, E472b, E472c, E472e) and total celluloses (E460, E461, E464, E466, E468). Emulsifiers with at least one statistically significant association with CVD risk are represented. Supplementary eTable2 provides the investigated associations between emulsifier intakes and CVD risk with corresponding hazard ratios and 95% confidence intervals. Multivariable Cox proportional hazard models were adjusted for age (timescale); sex; body mass index (continuous); physical activity (categorical International Physical Activity Questionnaire variable: high, moderate, low); smoking status (never smoker, former smoker, occasional smoker, regular smoker); number of smoked cigarettes in pack years (continuous); educational level (less than high school degree, <2 years after high school degree, ≥2 years after high school degree); number of dietary records (continuous); family history of CVD (yes/no); energy intake without alcohol (continuous, kcal/day); daily intakes of alcohol (continuous, g/day), saturated FAs (continuous, g/day), sodium (continuous, mg/day), total fibre (continuous, g/day), sugars (continuous, g/day), fruit and vegetables (continuous, g/day), red and processed meats (continuous, g/day), and whole grains (continuous, g/day); and proportion of ultra-processed food consumed in the diet, in weight (continuous, %). Standard deviations of emulsifier intakes (mg/day) were 3170.8 for total emulsifiers, 52.0 for total alginates, 35.3 for E401, 75.7 for total carrageenans, 73.2 for E407, 14.1 for E407a, 502.6 for total phosphates,58.4 for E339, 96.5 for E340, 227.2 for E341, 349.7 for E450, 122.3 for E451, 86.2 for E452, 93.4 for total celluloses, 69.4 for E460, 32.8 for E464, 32.0 for E466, 287.5 for total monoglycerides and diglycerides of FAs, 208.6 for E471, 103.7 for E472b, 57.6 for E472c, 28.3 for E472e, 63.5 for total polyglycerol esters of FAs, 61.5 for E475, 15.7 for E476, 23.1 for total lactylates, 22.7 for E481, 1147.1 for total modified starches, 78.3 for E322, 280.5 for E331, 69.7 for E410, 233.8 for E412, 428.1 for E414, 221.1 for E415, 310.6 for E440, 42.6 for E442, 2116.7 for E500, and 0.6 for E901. CVD=cardiovascular disease; FAs=fatty acids
After correction for potential multiple testing, all associations remained significant except for those between cellulose E460 and risk of coronary heart disease and CVD (both adjusted P=0.06), total monoglycerides and diglycerides of fatty acids and risk of cerebrovascular disease (adjusted P=0.1), and trisodium phosphate (E339) and risk of coronary heart disease (adjusted P=0.3). Overall, sensitivity analyses from models 1 to 6 (see supplementary eTable3) were consistent with results from the main models, and all statistically significant associations observed in this study went in the same direction in main and sensitivity analyses, suggesting a low risk of randomly significant associations. All observed associations with CVD risk remained significant when transient ischaemic attack and angina pectoris events were excluded from the CVD definition (severe CVD) (see supplementary eTable4).
Discussion
This prospective cohort study showed positive associations between higher intakes of total cellulose emulsifiers (specifically E460 and E466) and total monoglycerides and diglycerides of fatty acids (specifically E472b and E472c) and CVD risk. Higher intakes of total celluloses (specifically E460 and E466) and total monoglycerides and diglycerides of fatty acids (specifically E472c) as well as trisodium phosphate (E339) were positively associated with risk of coronary heart disease, and those of total monoglycerides and diglycerides of fatty acids (specifically E472b) were positively associated with risk of cerebrovascular disease.
The safety of food additive emulsifiers, as with all other food additives, is regularly assessed by authorities, such as the European Food Safety Authority in Europe, in comprehensive reports based on extensive literature evaluation, defining acceptable daily intakes when necessary. Based on the European Food Safety Authority’s latest evaluations, no acceptable daily intakes were deemed necessary to regulate the intakes of sodium citrate (E331),41 monoglycerides and diglycerides of fatty acids (E471),42 celluloses (E460, E461, E464, E466, E468),43 monoglycerides and diglycerides of fatty acids (E471),42 or lactic acid ester of monoglycerides and diglycerides of fatty acids (E472b).44 Although the acceptable daily intake for tartaric acid esters of monoglycerides and diglycerides of fatty acids was set at 240 mg/kg of body weight/day in 2020,44 none of the NutriNet-Santé study participants reached such intakes.7 Importantly, conclusions from European Food Safety Authority reports can only be drawn from the scientific evidence available at the time of evaluation. Nonetheless, the growing research interest in food additive emulsifiers14 led to novel and concerning findings from experimental work, which suggest a need for more regular evaluations assessing the safety of long term intakes at lower doses to these food additives, through individual or combined multi-intakes.
Comparison with other studies
This study explored and observed associations between the consumption of food additive emulsifiers and risk of CVD in a large group of adults over a long period. The current understanding about the effects of emulsifiers on health came from in vitro and in vivo experimental studies. For example, studies conducted on porcine small intestinal mucus showed that carboxymethylcellulose (E466) could damage the intestinal barrier, leading to intestinal inflammation.45 Similarly, high intakes of carboxymethylcellulose have been linked to changes in the composition of gut bacteria and increased risk of colon cancer.11464748 In a recent short term intervention study on humans, a supraphysiological dose of 15 g/day (compared with 3.9 mg/day in our study) of carboxymethylcellulose over 11 days increased markers of gut inflammation and reduced gut microbiota diversity compared with an additive-free diet.16 Similar pro-inflammatory effects have been observed with monoglycerides and diglycerides of fatty acids (E471) on faecal microbiota in vitro.49 However, experimental studies have suggested that carrageenan induced colitis could lead to a decrease in the population of Akkermansia muciniphila,5051 which may have protective effects against atherosclerosis.52 It is possible that disruptions in gut bacteria and increased gut inflammation could contribute to a systemic low grade inflammation that may affect gut health as well as other organs.53 In particular, imbalances in gut bacteria have been associated with metabolic and neurological conditions.54 Furthermore, in our study we observed positive associations between intake of celluloses and CVD risk. Although this might seem counterintuitive given the protective role of fibre on CVD,55 the finding could be linked to the disruption of food matrix in industrial products containing added celluloses compared with plants, which might lead to different effects on human health. Owing to the observational nature of our study, we were unable to confirm that emulsifiers are causally related to CVD risk. However, we have as much as possible isolated the role of emulsifiers by adjusting for the proportion of ultra-processed foods in the diet, as well as for several dietary features that might causally impact CVD risk, including intakes of sugar, sodium, saturated fatty acids, energy, fibre, and artificial sweeteners. Future short term human intervention studies, long term epidemiological studies, and preclinical experiments will bring additional arguments to strengthen the plausibility of causal associations.
Strengths and limitations of this study
The strengths of this study included its prospective design and large sample size. The NutriNet-Santé study was able to assess the intakes of food additives qualitatively and quantitatively with accuracy using detailed and repeated 24 hour dietary records, links to multiple food composition databases (OQALI,30 Open Food Facts,8 Global New Products Database,31 European Food Safety Authority, and Codex General Standard for Food Additives32), ad hoc laboratory assays, and dynamic matching to account for reformulations of industrial food items over time.7 Although more limited than in long term historical cohorts such as the Framingham study (20 years), the duration of follow-up (median 7.0 years, maximum 12.4 years) was similar to that of other cohort studies such as the UK Biobank,56 and to the duration of nutritional intervention studies on cardiovascular diseases prevention such as the Prevención con Dieta Mediterránea trial.57 In addition, the stability of the associations observed in this study over multiple sensitivity analyses suggest consistency and robustness of the findings.
Nonetheless, this study had some limitations, such as the high proportion of women in the cohort (79.3%), higher educational background, and overall more health conscious behaviours among the NutriNet-Santé study participants compared with the general French population, which may limit the generalisability of the results. This sex imbalance is common in volunteer based studies, especially in those linked to diet and health.58 The study is likely to have underestimated the strength of the observed associations because women tend to have healthier diets with lower emulsifier intakes (mean intake 4187 mg/day in women v 4509 mg/day in men, P<0.001) and a lower absolute risk of CVD. Moreover, ≈17% of the cohort was excluded owing to underreporting of energy intake assessed using a standard method,28 to eliminate true reporting errors in the absence of any restrictive diet. This proportion was consistent with the one observed in other studies—for example, 25.1% in the American National Health and Nutrition Examination Survey study59 and 18% in the Norwegian breast cancer screening programme.60 In the nationally representative Individual and National Studies on Food Consumption 3 study conducted in 2016 by the French Food Safety Agency,61 18% of adult participants were found to be under-reporting using the Black method applied in the present study. Moreover, even though dietary records were validated against blood and urinary biomarkers for energy and key nutrients, intake of emulsifiers has not been validated against blood or urine assays owing to lack of specific biomarkers. Besides, intakes might have been underestimated in food items exempt from food labelling (eg, bakery pastries), and non-additive originated emulsifiers occurring naturally in food products, such as lecithins in eggs, were not captured, because to our knowledge food composition databases that estimate their presence in foods are not available. Although these potential measurement errors may have biased the associations towards an unclear direction, this was more likely towards the null (non-differential errors due to the prospective design). In addition, some individual emulsifiers were consumed by an insufficient number of participants to be investigated individually. However, all available intakes of emulsifiers consumed were included in the calculation of exposure to total and groups of emulsifiers. Finally, residual confounding in the observed associations cannot be entirely ruled out, although this concern has been mitigated by using multivariable Cox models accounting for a wide range of potential confounders.
Policy implications and conclusions
Results from this large prospective cohort suggest that additive emulsifiers may be associated with an increased risk of CVD. These findings should be replicated in future epidemiological cohorts and mechanisms should be further elucidated by experimental approaches. Despite the moderate magnitude of the associations, these findings may have important public health implications given that these food additives are used ubiquitously in thousands of widely consumed ultra-processed food products. The results will contribute to the re-evaluation of regulations around food additive usage in the food industry to protect consumers. Meanwhile, several public health authorities recommend limiting the consumption of ultra-processed foods as a way of limiting exposure to non-essential controversial food additives.6263
What is already known on this topic
-
Emulsifiers are food additives widely used in industrially processed foods to improve texture and extend shelf-life
-
Research on healthy individuals suggests deleterious effects of food additive emulsifiers on the intestinal microbiota and metabolome
-
Such effects can lead to chronic intestinal inflammation and increasing susceptibility to carcinogenesis, and potentially cardiovascular disease (CVD)
What this study adds
-
Higher intakes of two emulsifier groups (total celluloses and total monoglycerides and diglycerides of fatty acids), and in particular four emulsifiers (E460, E466, E472b, E472c) were independently and positively associated with risk of CVD
-
These results suggest that food additive emulsifiers are associated with increased risk of CVD in humans
-
Given that these food additives are used ubiquitously in thousands of widely consumed ultra-processed food products, these findings have important public health implications