Cancer and neoplasms
Colon metastasis from pancreatic cancer
1Department of Gastroenterology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, 430030, People’s Republic of China; 2Institute of Pathology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, 430030, People’s Republic of China; 3Department of Medical Ultrasound, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, 430030, People’s Republic of China
Correspondence: Jingmei Liu, Department of Gastroenterology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, 430030, People’s Republic of China, Email [email protected] Jiqiao Liu, Department of Medical Ultrasound, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, 430030, People’s Republic of China, Email [email protected]
Abstract: Pancreatic cancer commonly metastasizes to the liver, lung or adrenal glands, but rarely spreads to the colon. We describe a case of a 65-year-old man with operation history of endoscopic submucosal dissection for rectal adenoma, who visited our department with a lesion in the sigmoid colon. A biopsy of the sigmoid pathologic lesion found heterologous cells in the muscularis mucosa, which indicated that this lesion did not originate in the colon. Abdominal enhanced CT results revealed a soft tissue mass in pancreatic tail and several masses in the liver and rectovesical pouch. 18-FDG PET-scan results showed pancreatic neoplastic mass. Biopsy result of pancreatic pathologic area was positive for ductal pancreatic adenocarcinoma. Immunohistochemical staining confirmed that the sigmoid lesion was a metastasis from a primary pancreatic adenocarcinoma—an unusual pattern of spread. The patient accepted chemotherapy after an oncologic evaluation. To our knowledge, there were only nine reported cases of metastatic pancreatic cancer spreading to the colon. This was a rare route of metastasis for pancreatic cancer. It is important to keep this possibility in mind when patients present with a colon lesion. Furthermore, our case highlights the importance of considering metastases when a colon mass is found in patients with a history of colon cancer, although primary colon cancer is still more likely.
Keywords: pancreatic cancer, colon cancer, sigmoid colon, colon metastasis, case report
Introduction
Pancreatic ductal adenocarcinoma (PDAC), generally known as pancreatic cancer, is one of the most aggressive forms of malignancy and is usually diagnosed at an advanced stage, with 12% of patients surviving for 5 years.1 As a typical chemotherapy drug, gemcitabine is the basis for the treatment of advanced pancreatic cancer, and a major role in the uptake of gemcitabine is played by human equilibrative nucleoside transporter 1 (hENT-1), which is a good biomarker for gemcitabine efficacy in PDAC.2 Two combination regimens, including 5-fluorouracil/leucovorin with irinotecan and oxaliplatin (FOLFIRINOX) and gemcitabine/nab-paclitaxel, can profoundly improve the prognosis of advanced pancreatic cancer.3,4 Besides, new progress has been made in the treatment of pancreatic cancer. Recent studies on highly aggressive PDAC cells confirm the ability of pyruvate dehydrogenase kinase (PDK) inhibitors to hamper PDK axis and to induce cancer cell death by apoptosis,5 and targeting focal adhesion kinase renders pancreatic cancers responsive to checkpoint immunotherapy.6
Prevention and control of cancer metastasis is one of the most important problems in cancer care. The most frequent sites of metastatic pancreatic cancer are liver, lung and adrenal glands.7 Metastasis of pancreatic cancer to the colon is extremely rare. To our knowledge, there are a few previously reported cases of pancreatic cancer metastasis to the colon.8–16 We reported a case of colon metastasis from pancreatic cancer in which this patient initially visited our hospital because of colon mass.
Case Report
A 65-year-old man visited his local hospital with lower abdominal discomfort, and 10-kg weight loss for several months. Because the patient had a history of rectal adenoma and underwent endoscopic submucosal dissection of the rectum lesion in 2017, a routine colonoscopy was performed by the local hospital. Colonoscopy results revealed a lesion in the sigmoid. Subsequently, he visited our hospital to perform further analysis.
On physical examination, his left abdomen was diffusely tender. The remainder of the physical exam was normal. Magnified narrow-band imaging endoscopy was performed to assess whether the colon lesion should be treated endoscopically or need surgical treatment. Magnified NBI endoscopy revealed an irregular mass in the sigmoid colon with sluggish peristalsis (Figure 1), and the microvascular architecture (capillary pattern) of this lesion was of IIIB type, which is observed in deep submucosal invasive carcinomas. Biopsy results revealed that glandular cells with atypia were seen in the mucosal muscularis of the sigmoid colon, and no clear adenomatous change was noted within the colonic mucosa, which is not consistent with the clinical pathological feature of primary colon cancer.
 |
Figure 1 (A) White-light endoscopy showed a slightly elevated lesion more than 10mm, with irregular surface structure and a demarcation line. (B) Magnify narrow-band imaging (NBI) endoscopy showed that the lesion was classified as capillary patterns (CP) type IIIB according to the NBI CP classification. |
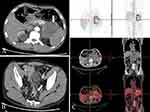
Furthermore, we performed a series of examinations to determine the origin of tumor. Neoplastic markers showed the elevated levels of gastrointestinal tumor markers carcinoembryonic antigen (CEA) and cancer antigen 72–4 (CA 72–4), which were 9.19 ng/mL (reference ≤5 ng/mL) and 21.7 U/mL (reference ≤6.9 U/mL), respectively. It is worth noting that cancer antigen 19–9 (CA 19–9), a useful marker for bile duct and pancreatic cancer, was significantly elevated, which was 2457 U/mL (reference ≤34 U/mL). Moreover, abdominal computed tomography (CT) showed a soft tissue mass (6.9*7.2cm) at the tail of pancreas (Figure 2). In addition, CT results also showed thickening of the sigmoid colon and several masses in the liver and rectovesical pouch (Figure 2). Endoscopic ultrasound (EUS) examination was performed along with fine-needle aspiration (FNA) of pancreatic mass. Biopsy of the pancreas results identified that glands were heterotypic, some of which had a cribriform pattern. Glands of typical colorectal morphology were not identified. 18-FDG PET-scan showed pancreatic neoplastic mass (6.2*7.2cm) with an obvious uptake of 18-FDG (SUVmax, 10.3) and a suspected secondary lesion in liver (SUVmax, 7.9) and rectovesical pouch (SUVmax, 5.8) (Figure 2). In addition, light sigmoid colon uptake (SUVmax, 4.6) was also revealed by 18-FDG PET. Furthermore, immunohistochemical stain was performed on the colonic lesion (Figure 3) and pancreatic lesion (Figure 4), which showed that these two lesions were strongly and diffusely positive for cytokeratin 7, was negative for cytokeratin 20. The histomorphology and immunohistochemical findings were supportive of a pancreatic primary and argued against a colonic primary, and the lesion in the sigmoid colon may be a metastatic lesion. All these features were consistent with the diagnosis of colon metastasis from pancreatic cancer. As such, the patient had a final diagnosis of stage IV pancreatic carcinoma, and he accepted chemotherapy with Gemcitabine and Abraxane I.V according the scheme day 1, 8, 15 of a 28-day cycle after an oncologic evaluation. This patient expressed his thanks to us for the right diagnosis and effective treatment.
|
Figure 2 (A) Contrast-enhanced CT showed a mass, 69*72mm in the tail of pancreas (White arrow). (B) Contrast-enhanced CT showed the thickening of the sigmoid colon (White arrow). (C) 18-FDG PET-scan showed a pancreatic metabolic lesion compatible with neoplastic mass. |
|
Figure 3 (A) Low power and (B) higher power image of hematoxylin and eosin stains showed the atypical glands in the mucosal muscularis of the sigmoid colon. (C and D) Immunohistochemical stains of the adenocarcinoma of the sigmoid colon showed strong positivity in CK7 (C) and negative in CK20 (D). |
|
Figure 4 (A) Low power and (B) higher power image of hematoxylin and eosin stains of the pancreatic mass showed atypical glands with a cribriform pattern. (C) Cytokeratin 7 expression within the glandular component was positive. (D) Cytokeratin 20 expression was negative within the glands. |
Discussion
Pancreatic cancer is a relatively uncommon malignancy. Most patients with pancreatic cancer are diagnosed at an advanced stage. The prognosis of pancreatic cancer is poor, with 5-year survival of 12%.1 Therefore, it is critical to identify the mechanisms of pancreatic cancer metastasis. There are a few possible routes of metastasis for pancreatic cancer, including serosal cavities in anatomical regions near primary cancer, lymphogenous routes, hematogenous routes and transcoelomic routes.17 Metastases of pancreatic cancer are usually located in the liver, lung, peritoneum or adrenal glands7,8 but are extremely infrequent in the colon.
In our case, it was extremely difficult to identify with certainty whether the colon lesion was a metastasis from the pancreas, or the pancreatic lesion was a metastasis from the colon, or synchronous primary cancers. The main feature of primary colorectal adenocarcinoma is neoplastic change of mucosal glands and invasion through the muscularis mucosae into the submucosa, usually with the presence of an adjacent precursor lesion (adenoma or serrated polyp). The absence of mucosal involvement or the presence of extensive mucosal–submucosal lymphatic involvement is strong evidence in favor of metastasis. In our case, no clear adenomatous change was noted within the colonic mucosa, indicating that the colon lesion may be a metastatic site.
In addition, the levels of some tumor markers were increased in our patient, especially CA19-9, which was elevated to 2457 U/mL at the time of admission. CA 19–9 is a common tumor marker, which expresses in cholangiocarcinoma, hepatocellular carcinoma, gastrointestinal cancer and pancreatic cancer.18 According to a recent study, sensitivity and specificity of CA 19–9 in the diagnosis of pancreatic cancer are 83.7% and 90.4%, respectively.18 Elevated levels of CA19-9 combined with pancreatic neoplastic mass in our case indicate a high risk of pancreatic cancer. Histological evidence is necessary for the diagnosis of pancreatic cancer. EUS-FNA is regarded as the best modality to obtain tissue from pancreas, with a sensitivity and specificity of 89% and 96%, respectively.19 EUS-FNA in our case revealed atypical cells in the pancreas, some of which had a cribriform pattern, indicating the possibility of pancreatic ductal adenocarcinoma.
Immunohistochemistry tests were performed to determine whether the colonic lesion was primary colon adenocarcinoma or metastasis from the pancreas. CKs are proteins of keratin-containing intermediate filaments found in epithelial tissues. The expression of subtypes of CKs in epithelial cells depends on the type of specific epithelium. CK7 is commonly expressed in gastric epithelium and hepatic-biliary-pancreatic ductal epithelium. In contrast, CK20 is almost expressed in all cases of adenocarcinomas from lower gastrointestinal tract. Therefore, CK7 in combination with CK20 is used as a panel to determine the primary origin when metachronous or synchronous colon cancers occur in conjunction with carcinomas of other sites. Most primary colon adenocarcinomas are generally CK7 negative and CK20 positive, whereas primary pancreatic cancers are almost CK7 positive and CK20 negative.20 Immunohistochemistry results showed the lesions were positive for CK7 and negative for CK20, favoring a pancreatic origin of the tumor. The final diagnosis of metastatic pancreatic cancer to the colon was not established until pathologic results of the colon lesion confirmed pancreatic adenocarcinoma.
According to PubMed databases, only nine cases of metastasis from pancreatic carcinoma to colon were described. The average age of patient was 65 years, with no sex difference. Most cases of the primary lesion originated from the tail of the pancreas. About half of the cases with metachronous colonic metastasis are from pancreatic cancer, and the longest time from pancreatic cancer diagnosis to colon metastases is 7 years. Sigmoid colon is the most common metastatic site, followed by ascending and transverse colon, and cecum metastasis from pancreatic cancer is reported in only one case. Patients with colon metastases from pancreatic cancer generally have a poor prognosis. The average survival time after detecting colon metastases is 7 months, with only one case surviving more than 1 year.
The great majority of cases are diagnosed postoperatively. In our case, it was diagnosed preoperatively, and the patient received the best management and treatment options. This was a rare route of metastasis for pancreatic cancer. It is important to keep this possibility in mind when patients present with a colon lesion. Furthermore, our case highlights the importance of considering metastases when a colon mass is found in patients with a history of colon cancer, although primary colon cancer is still more likely.
Ethics Approval and Consent to Participate
Ethical review and approval were not required for the study on human participants in accordance with local legislation and institutional requirements. The patient provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Acknowledgments
We gratefully thank all members of the Department of Gastroenterology, Tongji Hospital of Tongji Medical College, Huazhong University of Science and Technology for helping with this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; and gave final approval for the version to be published. All authors have agreed on the journal to which the article has been submitted, and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors declare no conflicts of interest.
References
1. Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17–48. doi:10.3322/caac.21763
2. Randazzo O, Papini F, Mantini G, et al. ”Open sesame?”: biomarker Status of the Human equilibrative nucleoside transporter-1 and molecular mechanisms influencing its expression and activity in the uptake and cytotoxicity of gemcitabine in pancreatic cancer. Cancers. 2020;13:12. doi:10.3390/cancers13010012
3. Zeng S, Pottler M, Lan B, Grutzmann R, Pilarsky C, Yang H. Chemoresistance in pancreatic cancer. Int J Mol Sci. 2019;20:4504. doi:10.3390/ijms20184504
4. Conroy T, Desseigne F, Ychou M, et al. Groupe tumeurs digestives of P. intergroup, FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825. doi:10.1056/NEJMoa1011923
5. Pecoraro C, De Franco M, Carbone D, et al. 1,2,4-Amino-triazine derivatives as pyruvate dehydrogenase kinase inhibitors: synthesis and pharmacological evaluation. Eur J Med Chem. 2023;249:115134. doi:10.1016/j.ejmech.2023.115134
6. Jiang H, Hegde S, Knolhoff BL, et al. Targeting focal adhesion kinase renders pancreatic cancers responsive to checkpoint immunotherapy. Nat Med. 2016;22:851–860. doi:10.1038/nm.4123
7. Kamisawa T, Wood LD, Itoi T, Takaori K. Pancreatic cancer. Lancet. 2016;388:73–85. doi:10.1016/S0140-6736(16)00141-0
8. Park DY, Krishnamurthi S, Chahal P, Downs-Kelly E, Morris-Stiff G. Pancreatic metastases to the colon: an unusual cause of colonic obstruction. BMJ Case Rep. 2019;12:e228578. doi:10.1136/bcr-2018-228578
9. Miyasaka M, Noji T, Tanaka K, Nakanishi Y, Asano T. Oncological emergency surgery for metachronous large and small bowel metastases after pancreaticoduodenectomy for pancreatic cancer: a case report. Surg Case Rep. 2018;4:99. doi:10.1186/s40792-018-0506-4
10. Gizzi G, Santini D, Golfieri R, Fuccio L. Diffuse colonic metastases from primary pancreatic cancer. Gastrointest Endosc. 2017;85:678–679. doi:10.1016/j.gie.2016.04.024
11. Kelley KM, Myer BS, Berger JJ. Malignant large bowel obstruction: a rare presentation of metastatic pancreatic cancer. Am Surg. 2016;82:e206–208. doi:10.1177/000313481608200813
12. Kim W, Lee Y. Metachronous colonic metastasis from pancreatic cancer presenting as mechanical obstruction: a case report. Clin Imaging. 2015;39:699–701. doi:10.1016/j.clinimag.2015.01.010
13. Inada K, Shida D, Noda K, Inoue S, Warabi M, Umekita N. Metachronous colonic metastasis from pancreatic cancer seven years post-pancreatoduodenectomy. World J Gastroenterol. 2013;19:1665–1668. doi:10.3748/wjg.v19.i10.1665
14. Ogu US, Bloch R, Park G. A rare case of metachronous skip metastasis of pancreatic cancer to the colon. Am Surg. 2012;78:E342–343. doi:10.1177/000313481207800704
15. Nogueira ST, Pinto BL, Silva EF, Garcia HA, Carneiro F. Pancreatic cancer presenting as colonic disease. A rare case report. Int J Surg Case Rep. 2018;44:4–7. doi:10.1016/j.ijscr.2018.01.019
16. Bellows C, Gage T, Stark M, McCarty C, Haque S. Metastatic pancreatic carcinoma presenting as colon carcinoma. South Med J. 2009;102:748–750. doi:10.1097/SMJ.0b013e3181a8fad7
17. Nakaya T, Oshiro H, Saito T, et al. Metastasis of pancreatic cancer within primary colon cancer by overtaking the stromal microenvironment. Int J Clin Exp Pathol. 2018;11:3141–3146.
18. Cwik G, Wallner G, Skoczylas T, Krzyzanowski M, Ciechainski A, Madro P. Elevated tumor marker CA 19-9 in the differential diagnosis of pancreatic mass lesions, Annales Universitatis Mariae Curie-Sklodowska. Medicina. 2004;59:213–218.
19. Puli SR, Bechtold ML, Buxbaum JL, Eloubeidi MA. How good is endoscopic ultrasound-guided fine-needle aspiration in diagnosing the correct etiology for a solid pancreatic mass?: a meta-analysis and systematic review. Pancreas. 2013;42:20–26. doi:10.1097/MPA.0b013e3182546e79
20. Chu P, Wu E, Weiss LM. Cytokeratin 7 and cytokeratin 20 expression in epithelial neoplasms: a survey of 435 cases. Mod Pathol. 2000;13:962–972. doi:10.1038/modpathol.3880175