Cancer and neoplasms
Current and Emergent Therapies for Systemic Mastocytosis
Systemic mastocytosis (SM) is a hematologic neoplasm characterized by clonal proliferation of mast cells in 1 or more organs including skin, bone marrow, gastrointestinal tract, spleen and/or liver.1 The underlying molecular pathogenesis of SM is an activating mutation in the KIT gene that leads to the constitutive proliferation of mast cells. In nearly 95% of patients, this is the KIT D816V mutation in exon 17; most other cases also demonstrate other mutations (including in other exons) of KIT.
Both the 5th edition of the World Health Organization classification of myeloid neoplasms and the International Consensus Classification of Myeloid Neoplasms organize mastocytosis into 2 broad types: cutaneous mastocytosis and SM.2,3 The former is mostly seen in children and the mastocytosis is confined to the cutaneous tissues only. SM, on the other hand, refers to the neoplastic mast cell infiltration of 1 or more extracutaneous tissues. These are frequently the bone marrow and gastrointestinal tract. There are 5 subtypes of SM: indolent SM (ISM) and smoldering SM (SSM), considered together as nonadvanced SM; aggressive SM (ASM); SM with an associated hematologic neoplasm (SM-AHN) or myeloid neoplasm; and mast cell leukemia (MCL), the last 3 considered together as advanced SM (~10% of the overall SM population).
Patients with SM can present with a variety of symptoms as well as the presence of B findings (high disease burden without organ dysfunction) and C findings (organ dysfunction from mast cell infiltration). These symptoms are related to release of mast cell mediators and organ infiltration. Patients with SM can have a significant symptom burden that substantially affects their quality of life.4,5
The survival outcomes of nonadvanced SM are excellent. In the 2019 analysis of the European Competence Network on Mastocytosis (ECNM) registry, patients with ISM (n=1639) had a median overall survival of 28.4 years.5 These outcomes are thought to be in line with that of the expected survival of the normal age- and sex-matched population.6 However, these patients may face a tremendous symptom burden and related negative consequences. The options have been limited to trigger avoidance, supportive care using drugs such as proton pump inhibitors, H1 and H2 blockers, leukotriene antagonists and mast cell stabilizers, and use of epinephrine pens for management of anaphylaxis.
For those with advanced SM, the outcomes are far worse. In the ECNM registry, those with ASM, SM-AHN, and MCL had median survival of 5.7 years, 2.9 years, and 1.6 years, respectively.5,7 This is despite treatment with the aforementioned medications and cytotoxic agents such as cladribine, as well as interferon.
However, the field is fortunately witnessing a paradigm shift with precision medicine in the form of mutant KIT inhibitors. At the 11th Annual Meeting of the Society of Hematologic Oncology (SOHO 2023), Prithviraj Bose, MD, professor of medicine at The University of Texas MD Anderson Cancer Center in Houston will discuss the diagnostic updates in systemic mastocytosis and current treatment landscape, including the targeted KIT inhibitors. Clinical trials that are ongoing in both nonadvanced and advanced forms of SM will be highlighted. This session is at 7:00 am on September 6, 2023.
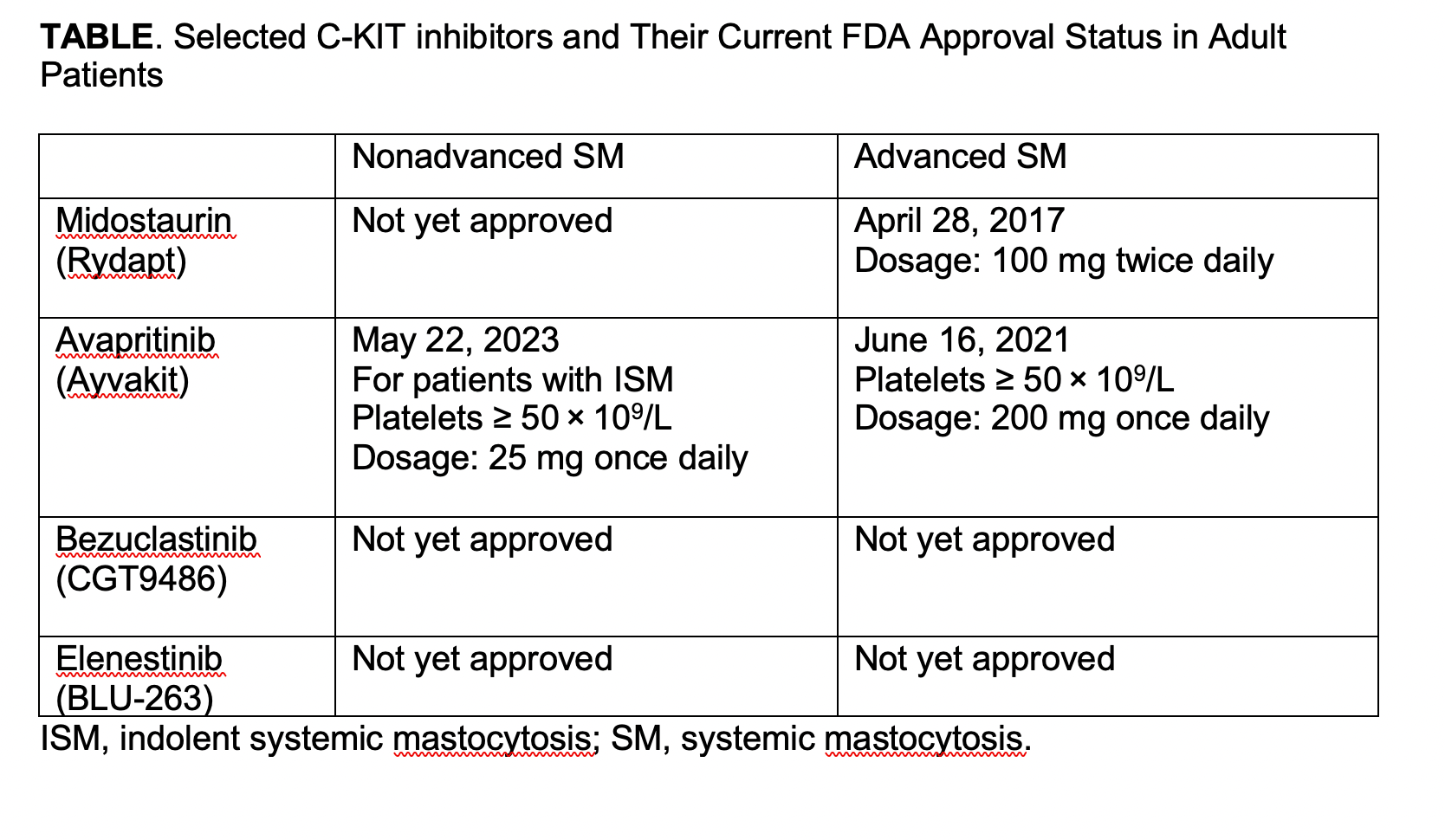
Midostaurin (Rydapt®; Novartis)
In an international, multicenter, single-arm, open-label, phase 2 study (CPKC412D2201; NCT00782067), midostaurin —a multikinase inhibitor—was administered at 100 mg twice daily in 116 patients with advanced SM.8 Using modified Valent criteria, the overall response rate (ORR) was 60% among the 89 patients evaluable for efficacy, but only 17% by International Working Group criteria and 28% when including clinical improvement; their median overall survival was 28.7 months.9 The median best-percentage changes in bone marrow mast cell burden and serum tryptase level were −59% and −58%, respectively. The most frequent adverse events were low-grade nausea, vomiting, and diarrhea. The findings led to a major shift in treatment approach from cladribine/interferon to KIT inhibition and formed the basis of regulatory approval of the drug. Subsequent retrospective analysis has shown improved outcomes (responses and survival) with midostaurin over cladribine.10
Avapritinib (Ayvakit™; Blueprint Medicines Corp)
Three important studies have led to the approval of avapritinib in patients with SM. The drug has selective activity against KIT D816V as well as PDGFRA; PDGFRA mutations; and KIT exon 11, 11/17, and 17 mutations.11
In the phase 1 dose escalation EXPLORER study (NCT02561988), avapritinib (30-400 mg) was administered to 86 adult patients with advanced SM, with expansion cohorts at 200 mg and 300 mg daily.12 By modified IWG-Myeloproliferative Neoplasms Research and Treatment and European Competence Network on Mastocytosis (MRT-ECNM) criteria, the ORR was 75% in response-evaluable patients. Of note, intracranial bleeding occurred in 13% overall, but in only 1% of patients without severe thrombocytopenia (platelets<50×109/L). A dosage of 200 mg daily was established as the recommended phase 2 dose based on efficacy and tolerability. This was investigated in the pivotal, single-arm, phase 2 PATHFINDER trial (NCT03580655).13 An ORR of 75% was again seen. Only 1 intracranial bleeding episode was noted in a patient with platelets less than 50×109/L. Dose-related, low-grade cognitive events were observed but did not result in substantial dose reductions. Reductions of 50% or more from baseline in serum tryptase (93%), bone marrow mast cells (88%), and KIT D816V variant allele fraction (60%) were observed. Importantly, in these studies, the responses were observed irrespective of the type of advanced SM, as well as prior use of midostaurin. Together, these studies led to the approval of avapritinib for patients with advanced SM. Improved outcomes and a better adverse event profile compared with midostaurin have led to avapritinib being preferred in patients with advanced SM.
The drug was also studied in PIONEER (NCT03731260), a phase 2, multipart, randomized, placebo-controlled, double-blind trial investigating avapritinib plus best supportive care in patients with symptomatic ISM.14
Advertisement
In part 1, a dosage of 25 mg once daily was recommended for part 2 and part 3. Part 2 enrolled 212 patients. The trial met its primary end point (mean total symptom score decrease). From baseline to week 24, patients treated with avapritinib had a decrease of 15.6 points vs 9.2 points in the placebo group (P<.003). Additional symptom benefits were noted beyond 24 weeks. The trial also met all its key secondary end points. Notably, a 50% or greater reduction in serum tryptase level was seen in 54% of patients taking avapritinib vs 0% (P<.001) in the placebo arm from baseline to week 24. Similarly, more patients in the avapritinib group had a 50% or greater reduction in KIT D816V VAF in peripheral blood (68% vs 6%; P<.001), 50% or greater reduction in total symptom score (TSS; 25% vs 10%; P=.005), 30% or greater reduction in TSS (45% vs 30%; P=.009), and 50% or greater reduction in bone marrow mast-cell burden (53% vs 23%; P<.001).
Best supportive care usage decreased or was discontinued in more patients who were taking avapritinib. Importantly, these gains translated to an improved quality of life using various instruments (SF-12, SF-12 mental health component score, EQ-VAS, MC-QoL). The drug was very well tolerated. Mild (grade 1, 93%) and relatively few cases of edema and alkaline phosphatase increase were noted in the avapritinib arm. No intracranial bleeding episodes occurred. These results have led to avapritinib being the only treatment specifically approved for patients with ISM. The study is ongoing and following patients in the extension phase. In part 3, patients receiving placebo crossed over to the avapritinib arm after 24 weeks of blinded treatment in part 2.
Bezuclastinib (CGT9486)
Bezuclastinib (Cogent Biosciences) is an oral, highly selective, and potent tyrosine kinase inhibitor (TKI) with specificity for mutations in KIT exons 9, 11, 17, and 18, including D816V. It spares closely related kinases (eg, PDGFRα, PDGFRβ, and CSF1R) and only minimally crosses the blood-brain barrier, which may potentially help prevent intracranial bleeding and cognitive adverse events.
Early data presented at the 2022 American Society of Hematology Annual Meeting and Exposition on 11 mIWG-evaluable patients enrolled in APEX (NCT04996875), a phase 2, open-label, 2-part, multicenter study in adult patients with advanced SM, suggest that the drug is well-tolerated and showed overall response rates similar to those seen with avapritinib. The drug is also being investigated vs placebo in patients with ISM or SSM in the phase 2 SUMMIT trial (NCT05186753).
Elenestinib (BLU-263; Blueprint Medicines Corp)
Elenestinib is a next-generation, highly selective, KIT D816V inhibitor that is minimally brain-penetrant, similar to bezuclastinib. It is under investigation in HARBOR (NCT04910685), a randomized, double-blind, placebo-controlled, phase 2/3 study in ISM and a small cohort of patients with monoclonal mast cell activation syndrome.
Top-line, 12-week results from the dose-finding part 1 study showed improvement in TSS across dose cohorts and objective markers of mast cell burden, as well as KIT D816V VAF. It is also being investigated in AZURE (NCT05609942), a phase 1/2, open-label, 2-arm study evaluating elenestinib as monotherapy and in combination with azacitidine, in patients with KIT-altered hematologic malignancies.
Future Outlook
The arrival of potent C-KIT inhibitors (TABLE) has ushered in a new area in the management of SM. Patients with advanced SM now have a targeted therapy available that provides more frequent and deeper remissions. Ongoing studies evaluating next generation C-KIT inhibitors will help address if a combinatorial or sequential approach with other therapies such as hypomethylating agents would help even more in preventing progression and inducing remissions while maintaining safety. The role of transplant in the era of potent C-KIT inhibitor therapy needs to be reanalyzed and assessed. On the other hand, for those with nonadvanced SM, the approval of avapritinib is a major win that improves symptoms and quality of life and possibly modifies the underlying disease biology. It will be important to demonstrate its long-term safety and tolerability in patients with ISM, and newer options for those who do not respond or lose response will be much welcome.