Blood
Red Blood Cell Transporter Finding May Lead to New Drugs for Blood Disorders
Researchers at the Icahn School of Medicine at Mount Sinai and collaborators say they have identified the structure of a special transporter found in red blood cells and how it interacts with drugs. Details on the findings (“Substrate binding and inhibition of the anion exchanger 1 transporter”), which were reported in Nature Structural & Molecular Biology, could lead to the development of more targeted medicines, according to the team.
The research team found that this transporter facilitates the movement of a bicarbonate, which certain drugs can inhibit. They discovered how these drugs block the transporter and devised novel compounds capable of achieving the same effect.
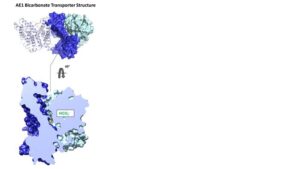
“Anion exchanger 1 (AE1), a member of the solute carrier (SLC) family, is the primary bicarbonate transporter in erythrocytes, regulating pH levels and CO2 transport between lungs and tissues. Previous studies characterized its role in erythrocyte structure and provided insight into transport regulation. However, key questions remain regarding substrate binding and transport, mechanisms of drug inhibition and modulation by membrane components,” write the investigators.
“Here we present seven cryo-EM structures in apo, bicarbonate-bound and inhibitor-bound states. These, combined with uptake and computational studies, reveal important molecular features of substrate recognition and transport, and illuminate sterol binding sites, to elucidate distinct inhibitory mechanisms of research chemicals and prescription drugs. We further probe the substrate binding site via structure-based ligand screening, identifying an AE1 inhibitor. Together, our findings provide insight into mechanisms of solute carrier transport and inhibition.”
“Our findings provide a detailed understanding of how bicarbonate transporters work, and the newly identified tool compounds open doors to studying conditions involving red blood cells, including hemolytic anemias,” says Daniel Wacker, PhD, corresponding author and an assistant professor of pharmacological sciences, neuroscience, and genetic and genomic sciences at Icahn Mount Sinai.
Previously, notes Wacker, human bicarbonate transporters were poorly understood, despite being involved in many aspects of human physiology, including regulating pH that involves keeping the level of acidity within a specific range.
Using cryo-electron microscopy, the team identified high-resolution structures revealing bicarbonate and inhibitor binding, and their impact on the transport mechanism. The scientists then used computer simulations to analyze millions of compounds that could interact with the substrate binding site.
Their experiments pinpointed a group of innovative chemical inhibitors specifically designed for anion exchanger 1, a protein that is crucial for maintaining the proper function of the blood and red blood cells.
“Our study also demonstrates the potential for developing new inhibitors with medical potential for other solute carrier (SLC) proteins, a protein family gaining importance in drug development,” adds co-author Bin Zhang, PhD, the Willard T.C Johnson Research Professor of Neurogenetics and Director of the Mount Sinai Center for Transformative Disease Modeling at Icahn Mount Sinai.
Next, the researchers plan to expand their studies to other SLC proteins involved in a variety of disorders including neurodegenerative diseases, psychiatric maladies, and cancer.
“This study effectively paves the way to using atomic-level insights toward the rapid development of promising drug-like molecules for SLC proteins,” says co-author Avner Schlessinger, PhD, associate professor of pharmacological sciences and associate director of the Mount Sinai Center for Therapeutics Discovery at Icahn Mount Sinai.
Researchers from the University of California-Berkeley, and Amgen were other co-authors.