Cardiovascular
Diagnosis of pneumonia, sepsis after cardiovascular surgery
Introduction
Early postoperative bacterial pneumonia and sepsis (ePOPS), which occurs within the first 48 hours after cardiac surgery, is a common serious and life-threatening complication.1,2 The consequences of ePOPS are often devastating, dramatically increasing mortality, length of stay and medical costs for surviving patients.3–5 Indeed, patients developing ePOPS have a 10- to 17-fold increased odds of mortality, relative to those patients not.1,6–8
Compared with other surgical procedures, cardiovascular surgery is more traumatic, more bleeding/transfusion, and is more likely to face ischemia-reperfusion injury and cardiopulmonary bypass (CPB), resulting in severe inflammatory responses.9 A severe postoperative inflammatory response increases the level of infectious biomarkers, including white blood cells (WBC), procalcitonin (PCT), interleukin-6 (IL-6), and C-reactive protein (CRP) remains unclear.10–13 It may mask a patient’s clinical manifestation and cause difficulties in predicting ePOPS. At the same time, the culture of pathogens, which is a gold standard for diagnosis of infection, has its natural defects, such as low positive rates and a 2-to-5-day delay. It may delay the timing of postoperative adjustment of antibiotics.
Current clinical guidelines recommend the use of prophylactic antibiotics within the first 48 hours after cardiovascular surgery.14,15 However, there is insufficient evidence-based medical evidence to determine whether antibiotics should be continued thereafter.14,15 In the absence of effective risk assessment, prolonged antibiotic use may lead to overuse of antibiotics on the one hand, and underuse of antibiotics in some patients on the other hand. In particular, more than half of postoperative bacterial pneumonia and sepsis occur within 48 h after cardiovascular surgery. We, therefore, particularly need a predicting model, to guide clinicians on discontinuing, continuing or escalating antibiotics.
Therefore, the present study aimed to develop a novel predictive model for the diagnosis of ePOPS, with the hope that it could be applicable to guide the adjustment of antibiotics in the first 48 hours after surgery.
Methods
Source of Data
This study report conformed to the Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis (TRIPOD) statement.16 The data for this study were obtained from a database designed to develop a predictive model for the diagnosis of infection after cardiovascular surgery (DICS).17
From October 2020 to October 2021, a total of 1203 patients were included in the DICS database, and all participants were included in our study. Patients between 18 and 80 years of age scheduled for open heart surgery were eligible. Exclusion criteria included: 1. preoperative temperature ≥ 38°C; 2. patients undergoing cardiovascular surgery for trauma, infective endocarditis, tumors, and malignancies; 3. patients with preoperative infections; 4. patients with a diagnosis of inflammatory immune disease or connective tissue disease; 5. pregnant or breastfeeding; 6. patients who elected not to participate the informed consent form.
Study Outcomes
Postoperative bacterial pneumonia is diagnosed when a patient meets both clinical and bacteriologic strategies after surgery. (1): Clinical strategy: Emergence of a new or progressive radiographic infiltrate combined with two out of three clinical features (fever above 38°C, leukocytosis or leukopenia, and purulent secretions).18,19 (2): Pathogenic bacteria were detected twice in sputum bacteria culture. Sputum specimens were obtained using fiberoptic bronchoscope or alveolar lavage fluid when the patient was unable to cough up sputum voluntarily.
Postoperative sepsis is diagnosed when the patient’s blood culture is positive for bacteria after surgery and contamination is excluded.20
ePOPS was the primary outcome of this study and was defined as postoperative pneumonia or sepsis occurring within the first 48 hours after surgery; therefore, we report the time of the first positive specimen collection for sepsis in a patient with postoperative pneumonia. The secondary outcome of this study was infection-related adverse outcome (IRAO), defined as ePOPS patients hospitalized for more than 30 days or dying during hospitalization.
Pathogenic species were ranked and reported according to the number of pathogenic bacteria found in sputum and blood of ePOPS and IRAO patients.
Predictors
Factors identified in both the previous literature as possible indicators of ePOPS and those based on the clinical experience of surgeons and physical therapists were measured.
Each patient will undergo a standard clinical assessment at admission. The recorded clinical variables will be used for model development, including age, gender, height and weight to obtain body mass index (BMI), smoking status, drinking status, hypertension, diabetes, chronic lung disease, chronic renal insufficiency, liver insufficiency, previous surgery, etc. We will also record the details of the patient’s surgeries and complications, as well as the use of various instruments, including surgical procedures, surgical incision, cardiopulmonary bypass (CPB) time, aortic cross clamp (ACC) time, deep hypothermia circulatory arrest (DHCA), intraoperative blood transfusion volume and type, the amount and type of intraoperative drugs, use of extracorporeal membrane oxygenation (ECMO) or Intra-aortic Balloon Pump (IABP) or continuous renal replacement therapy (CRRT), etc.
Biomarker Measurements
Blood samples were collected using standardized procedures and tested for PCT, IL-6, and CRP immediately at 6:00 a.m. on the first day after the patient’s admission and on the first 3 days postoperatively.
The concentrations of the biomarkers (PCT, CRP and IL-6) were measured by cyclic enhanced fluorescent immunoassays (CEFA) performed on a fully automated, benchtop Pylon 3D immuno-analyzer (ET Healthcare, China) using whole blood samples. A description of CEFA assay procedures is available elsewhere.21
Other biomarkers, including leukocyte and neutrophil ratios, have not shown desirable diagnostic value in previous studies, but we have nevertheless included a number of variables that may be of value, as follows:22 White blood cell counts, Neutrophil ratio, Hematocrit, Platelet, Troponin T/I, albumin, Creatinine, B-type natriuretic peptide, myoglobin, D-dimer. We tested these serological indicators for the first time within 24 hours after the patient is admitted to the hospital, and the third day after surgery.
Chest Radiograph
Enrolled patients underwent bedside chest radiographs at least once a day until the third day after surgery. A chest radiograph review team consisting of two trained physicians was formed to review these chest radiographs individually and categorize them into 3 grades according to the severity of pulmonary leakage. Mild indicated chest films with no visible leakage, moderate included chest films with spotty or flaky leakage, and severe meant large exudates fused into pieces.
Missing Data
The potential bias of missing data was assessed by making comparisons between the characteristics of patients with contexts in which one or more predictor variables were missing and those for which complete data were available. All predictors containing more than 5% missing values were excluded (preoperative troponin T was excluded). Moreover, missing data were assumed to be missing at random and imputed using multiple imputation with chained equations. Multiple imputation was performed through the “mice” package in R.23 A total of twenty-five imputed datasets were generated, and the final inference estimations were combined using Rubin’s rules.
Model Development and Validation
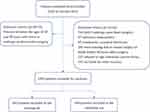
The entire dataset was randomly split 7:3 into training and validating sets (Figure 1). The training set was used for model development, while the validation set was used for model validation. The Least Absolute Shrinkage and Selection Operator (LASSO) based on the “glmnet” package was used to screen variables.24 The screened factors were further used for backward stepwise multivariate logistic regression analysis to identify independent risk factors. Based on the multivariate model, a nomogram was then constructed to assess the risk of ePOPS.
|
Figure 1 Flow chart showing the selection criteria for inclusion and exclusion and randomly dividing the dataset into training and test sets in a 7:3 ratio. |
The area under the receiver operating characteristic (ROC) curve and the area under the curve (AUC) were used to assess the discrimination. The calibration of the model was assessed using the Hosmer–Lemeshow goodness-of-fit (GOF) test. Ten-fold cross-validation was used for internal validation. External validation took place in the validation set.
Clinical Utility
First, we scored all patients using the nomogram obtained from the DICS-I model. Second, we plotted a ROC curve with score as the independent variable and the occurrence of POPS as the dependent variable, and then categorized the patients into low-risk and moderate-high risk groups based on the Youden index and the calculated optimal threshold. Subsequently, another ROC curve was plotted using the score of patients in the moderate-high risk group as the independent variable and whether IRAO occurred as the dependent variable, and then categorized the moderate-high risk group into moderate risk and high-risk groups according to the optimal threshold value calculated by Youden index. So far, we categorized the patients into low-risk group, moderate-risk group and high-risk group.
In practice, limited by the lack of accurate diagnostic tools, physicians often rely on clinical experience to determine whether a patient has developed a bacterial infection after cardiac surgery that requires prolonged antibiotic use or a change to a higher-grade antibiotic. We compared the risk of ePOPS in patients as judged by the DICS-I model with patients at high risk of bacterial infection as judged empirically by physicians in clinical practice. In order, to argue whether the DICS-I model can more accurately identify patients with ePOPS.
Statistical Analysis
The analyses were carried out with R version 4.1.2. Categoric variables were summarized as frequencies (%) and compared by χ2 or Fisher’s exact test. Continuous variables were expressed as median (interquartile range (IQR)). The Mann–Whitney U-test was used for comparison.
Results
During the study period, a total of 2819 patients aged 18 to 80 years who intended to undergo heart surgery were presented to our center, of whom 1616 were excluded (760 did not undergo open-heart surgery; 97 infectious endocarditis; 65 community-acquired infections; 281 were missing due to rescue surgery or death within 48 hours after surgery; 221 refused to sign informed consent forms; 192 excluded for other reasons). Finally, 1203 patients were enrolled and randomly divided into the training and validation sets in a 7:3 ratio (Figure 1). The average age of the enrolled patients at admission was 57.9±12.7 years, and more than half (n = 731, 60.8%) were male. There were no statistically significant differences in all indicators between patients in the training and validation sets after allocation by randomization (Table 1).
|
Table 1 Baseline Features of Training and Validation Datasets. Data are n (%) or Median (IQR) |
Outcomes
During the study period, 92 patients had postoperative pneumonia only, 11 patients had sepsis only, and 15 patients suffered from both. 87.3%(103/118) of postoperative pneumonias and sepsis occurred earlier than the morning of the third postoperative day, including 95 cases of pneumonia, 5 cases of sepsis, and 3 patients with both (Figure 2).
|
Figure 2 The time at which the bacterial pneumonia or sepsis occurred. |
Patients with ePOPS had a significantly higher risk of death and significantly longer duration of mechanical ventilation, intensive care unit stay and hospitalization compared to controls (Table 2). During the study period, a total of 34 cases of IRAO occurred, of which 14 died and 20 were hospitalized for longer than 30 days and eventually survived (Table 2).
 |
Table 2 Clinical Outcomes of Patients in the ePOPS and Control Groups |
Klebsiella pneumoniae was the most common pathogen, seen in 51.5% of ePOPS patients, of which over 70% had a favorable prognosis. However, Acinetobacter baumanii, Pseudomonas aeruginosa, and Enterobacter cloacae had a poor prognosis in nearly 50% of the cases (Table 3).
|
Table 3 Common Pathogens in Patients with ePOPS or IRAO |
Development of the Nomogram Model
Lambda = 0.02082464 was selected as the minimum criterion for the Lasso regression after 10-fold cross-validation of the Lasso coefficient profiles of 90 characteristics. After variable selection using the LASSO penalty, a total of ten variables remained, including mechanical ventilation duration, NYHA classification ≥III, diabetes on insulin, history of myocardial infarction, use of extra corporeal membrane oxygenation or intra-aortic balloon pump or continuous renal replacement therapy, chest radiograph exudates, cardiac troponin I on admission, B-type natriuretic peptide at POD3, IL-6 on POD3 and aggravated interleukin-6 (defined as continuously elevated interleukin-6 after surgery). These variables were included in the multivariate logistic regression, and finally duration of mechanical ventilation (OR: 1.02, 95% CI 1.00–1.04, P=0.015), NYHA classification ≥III (OR: 2.81, 95% CI 1.34–6.65, P=0.001), diabetes on insulin (OR: 3.17, 95% CI 1.70–5.79, P<0.001), exudation on chest radiograph (OR: 4.41, 95% CI 1.33–11.99, P=0.011) and IL-6 level on POD3 (OR: 1.00, 95% CI 1.00–1.01, P<0.001) were independent risk factors for the diagnosis of ePOPS (Table 4). A nomogram for the diagnosis of ePOPS was established based on LASSO logistic regression model, named DICS-I (Figure 3, Table 5).
|
Table 4 Multivariate Logistic Regression |
 |
Table 5 The Corresponding Scores of Different Indicators and the Probability of ePOPS Corresponding to the Total Score Were Calculated |
 |
Figure 3 A nomogram was drawn based on LASSO logistic model and named DICS-I. |
Model Discrimination and Calibration
The AUC of DICS-I model was 0.787 (95% CI: 0.726–0.848) in the training set and 0.739 (95% CI: 0.642–0.836) in the validation set (Figure 4a). By using 10-fold cross-validation, the accuracy of DICS-Is model was 0.805. The Hosmer–Lemeshow goodness-of-fit test showed that P=0.927 in the training dataset and P=0.184 in the validation dataset.
 |
Figure 4 (a): Performance of the DICS-I model for diagnosing ePOPS in training set and validation set; (b): Performance of the DICS-I model for diagnosing ePOPS and predicting IRAO in the full dataset. |
Clinical Utility
Based on the occurrence of ePOPS and IRAO, the optimal thresholds were set at 89 and 128 points, respectively, and patients were categorized into low, moderate, and high risk groups (Figure 4b). The three groups contained 850, 287 and 66 patients, respectively (Figure 5a). Of these, 50% (33/66) of the 66 patients in the high-risk group were confirmed as ePOPS cases (Figure 5a). Meanwhile, based on clinical experience, a total of 143 patients were upgraded with antibiotics within 48 hours postoperatively, but this covered only 19.4% (20/103) of ePOPS cases (Figure 5b). Surprisingly, 67.6% (23/34) of IRAO cases were included in the high-risk patient group identified by the DICS-I model (Figure 5c). In contrast, based on clinical experience, only 32.4% (11/34) of IRAO cases were identified (Figure 5d).
|
Figure 5 Clinical application of the DICS-I model versus escalation of antibiotics based on clinical experience. (a): DICS-I model subgroups and number of ePOPS patients in each group; (b): Percentage of ePOPS patients identified based on clinical experience; (c): IRAO cases identified in the DICS-I model groups; (d): Patients with IRAO identified based on clinical experience. |
Discussion
The value of peri-operative indicators for constructing the DICS-I model was evaluated in this cohort study. DICS-I model could effectively predict the risk of ePOPS in our study cohort. In addition, the 5-variable model was more effective in identifying high-risk patients, especially those prone to IRAO, compared with clinical experience. Based on the DICS-I model, we suggested clinicians escalate antibiotics when patients were identified as moderate- and high-risk of ePOPS.
According to the guidelines, prophylactic cefuroxime sodium and cefazolin sodium are the routine recommendation, and prolonged antibiotics without restriction may be harmful.14,15,25 Gram-negative bacilli, including Klebsiella pneumoniae, Pseudomonas aeruginosa and Acinetobacter baumannii, are the most common causative species of ePOPS after cardiovascular surgery in our center. Therefore, the escalated antibiotics, including cefperazone-sulbactam, piperacillin-tazobactam or imipenem cilastatin sodium, are appropriate.
In our study cohort, the vast majority of ePOPS occurred within approximately 48 h after surgery (103/119, 86.6%). Therefore, it may be of special significance that we assess the risk of ePOPS in patients at this particular time point, the morning of the POD3. In contrast, the DICS-I model identified 66.02% (68/103) ePOPS patients in the high-risk group, while the clinical experience group identified only 19.42% (20/103) ePOPS patients (66.0% vs 19.4%, P<0.001). The DICS-I model successfully identified 32 out of 34 patients with IRAO in the moderate-high risk group, while the clinical experience group identified only 11 out of 34 patients with IRAO (94.1% vs 32.4%, P<0.001). A considerable number of low-risk patients in the clinical experience group received escalation of antibiotic therapy, and the final diagnosis also proved that only one of these patients had ePOPS and no patient developed IRAO. Application of the DICS-I model score may help to reduce the misuse of antibiotics in these patients.
A considerable number of low-risk patients in the clinical experience group received antibiotic escalation 9.6% (82/850), and accounted for more than half of the patients in the clinical experience group 57.3% (82/143), but the final diagnosis also proved that only one of these patients had ePOPS and no patient developed IRAO. Therefore, the application of DICS-I score may help to reduce the risk of misuse of antibiotics in low-risk patients when antibiotics are escalated based on clinical experience. In other words, our study indicated that the DICS-I model could accurately predict ePOPS; the Gram-negative bacilli were common causative species of ePOPS. Moreover, first- or second-generation cephalosporins that might not cover the G(-) bacilli were recommended to be prophylactically administrated before and after cardiovascular surgery. We, therefore, strongly suggest escalating antibiotics when patients were identified as moderate- and high-risk of ePOPS.
Several previous studies have similarly focused on predictive models for pneumonia or sepsis after cardiac surgery, but no literature has focused on early postoperative period as a specific time point. A multicenter retrospective study in 2021, which enrolled 13,380 patients, utilized patient preoperative and intraoperative factors to predict the risk of pneumonia in patients after open cardiac surgery. This study culminated in the construction of a prognostic predictive model with 10 factors, which helped us to predict the risk of postoperative pneumonia in patients in the immediate postoperative period.26 Another earlier study constructed a risk prediction model for postoperative pneumonia with a total score of 33 by including age >65 years, chronic lung disease, peripheral arterial disease, cardiopulmonary bypass time >100 minutes, intraoperative red blood cell transfusion, and pre- or intraoperative intra-aortic balloon pump.27 One of the limitations considered in this study was the failure to assess the effect of surgical incision and ejection fraction on postoperative pneumonia, which was taken into account in our study due to its prospective design. Unlike previous predictive models, our study resulted in a diagnostic model that is more suitable for early postoperative diagnosis of whether a patient already has ePOPS, and more convenient for clinicians to adjust the antibiotic regimen at the right time.
In conclusion, according to our constructed DICS-I model, the risk of ePOPS in patients can be assessed early after surgery, and the higher the score, the higher the risk of IRAO. We suggested that patients in moderate- and high-risk groups needed to consider increasing the intensity of antibiotics, and that patients in the low-risk group may not need to upgrade antibiotics until aetiological evidence is available. However, all these conjectures need to be verified by more rigorous prospective, large-scale, multi-center clinical trials, and we are also working hard on this next-generation model.
Limitations and Future Directions
There are several limitations of our study. First, selection bias cannot be excluded because the data for this study were obtained from a single-center database. Second, The diagnostic model does not differentiate between infections of different severities and could be further improved in the future. Third, for emergency surgery, we could not ensure that community-acquired pneumonia was completely ruled out, which may have resulted in selection bias. Finally, we did the validation in patients from the same hospital, which may limit its generalizability.
Conclusion
The DICS-I model constructed in this study can be used to predict the risk of bacterial pneumonia and sepsis in the first 48 hours after cardiovascular surgery, and is also especially suitable for predicting the risk of IRAO in patients. Adjusting the use of antibiotics according to the DICS-I model may reduce the abuse of antibiotics and avoid the poor prognosis of some patients due to the inadequate use of antibiotics.
Abbreviations
ePOPS, early Postoperative bacterial Pneumonia and Sepsis; LASSO, Least Absolute Shrinkage and Selection Operator; ROC, the Receiver Operating characteristic Curve; AUC, the Area Under the receiver operating characteristic Curve; POD, Postoperative Day; DICS, Diagnosis of Infection after Cardiovascular Surgery; NYHA, the New York Heart Association; IL-6, InterLeukin-6; CPB, Cardiopulmonary Bypass; WBC, White Blood Cells; PCT, Procalcitonin; CRP, C-Reactive Protein; TRIPOD, the Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis statement; CEFA, Cyclic Enhanced Fluorescent immunoassay; GOF, the Hosmer–Lemeshow goodness-of-fit test; IQR, Interquartile Range; OR, Odds Ratio; CI, Confidence Interval; IRAO, Infection-Related Adverse Outcome.
Data Sharing Statement
Readers could access the original data (http://www.medresman.org.cn/pub/cn/proj/projectshshow.aspx?proj=4148).
Ethics Approval and Consent to Participate
Ethical approval was obtained from Medical Ethics Committee of Affiliated Nanjing Drum Tower Hospital, Nanjing University Medical College, in accordance with the principles of the Declaration of Helsinki on September 23, 2020 (2020-249-01). All included patients were required to provide written informed consent.
Consent for Publication
All the authors approved the publication of the manuscript.
Acknowledgments
The trial was registered at Chinese Clinical Trial Register (www.chictr.org.cn, ChiCTR2000038762, Registered September 30, 2020).
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
1. The Cardiovascular Disease Rescue Life Circle Project in Ili River Valley (Phase I): The No. 52 (2021) document issued by counterpart office of Jiangsu province. 2. The Clinical Medical Center of Nanjing Cardiovascular Diseases (CZLB506-2020).
Disclosure
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
1. Massart N, Mansour A, Ross JT, et al. Mortality due to hospital-acquired infection after cardiac surgery. J Thorac Cardiovasc Surg. 2020;163(6):2131–2140.e3. doi:10.1016/j.jtcvs.2020.08.094
2. Fowler VG, O’Brien SM, Muhlbaier LH, et al. Clinical predictors of major infections after cardiac surgery. Circulation. 2005;112(9 Suppl):I358–l365. doi:10.1161/CIRCULATIONAHA.104.525790
3. Greco G, Shi W, Michler RE, et al. Costs associated with health care-associated infections in cardiac surgery. J Am Coll Cardiol. 2015;65(1):15–23. doi:10.1016/j.jacc.2014.09.079
4. Gulack BC, Kirkwood KA, Shi W, et al. Secondary surgical-site infection after coronary artery bypass grafting: a multi-institutional prospective cohort study. J Thorac Cardiovasc Surg. 2018;155(4):1555–62 e1. doi:10.1016/j.jtcvs.2017.10.078
5. Ailawadi G, Chang HL, O’Gara PT, et al. Pneumonia after cardiac surgery: experience of the National Institutes of Health/Canadian Institutes of Health Research Cardiothoracic Surgical Trials Network. J Thorac Cardiovasc Surg. 2017;153(6):1384–91 e3. doi:10.1016/j.jtcvs.2016.12.055
6. Strobel RJ, Liang Q, Zhang M, et al. A preoperative risk model for postoperative pneumonia after coronary artery bypass grafting. Ann Thorac Surg. 2016;102(4):1213–1219. doi:10.1016/j.athoracsur.2016.03.074
7. Gelijns AC, Moskowitz AJ, Acker MA, et al. Management practices and major infections after cardiac surgery. J Am Coll Cardiol. 2014;64(4):372–381. doi:10.1016/j.jacc.2014.04.052
8. Chen LF, Arduino JM, Sheng S, et al. Epidemiology and outcome of major postoperative infections following cardiac surgery: risk factors and impact of pathogen type. Am J Infect Control. 2012;40(10):963–968. doi:10.1016/j.ajic.2012.01.012
9. Ng KT, Van Paassen J, Langan C, et al. The efficacy and safety of prophylactic corticosteroids for the prevention of adverse outcomes in patients undergoing heart surgery using cardiopulmonary bypass: a systematic review and meta-analysis of randomized controlled trials. Eur J Cardiothorac Surg. 2020;57(4):620–627. doi:10.1093/ejcts/ezz325
10. Li Q, Zheng S, Zhou PY, et al. The diagnostic accuracy of procalcitonin in infectious patients after cardiac surgery: a systematic review and meta-analysis. J Cardiovasc Med. 2021;22(4):305–312. doi:10.2459/JCM.0000000000001017
11. Adamik B, Kubler-Kielb J, Golebiowska B, et al. Effect of sepsis and cardiac surgery with cardiopulmonary bypass on plasma level of nitric oxide metabolites, neopterin, and procalcitonin: correlation with mortality and postoperative complications. Intensive Care Med. 2000;26(9):1259–1267. doi:10.1007/s001340000610
12. Opal SM, Wittebole X. Biomarkers of Infection and Sepsis. Crit Care Clin. 2020;36(1):11–22. doi:10.1016/j.ccc.2019.08.002
13. Jukic T, Ihan A, Stubljar D. Dynamics of inflammation biomarkers C-reactive protein, leukocytes, neutrophils, and CD64 on neutrophils before and after major surgical procedures to recognize potential postoperative infection. Scand J Clin Lab Invest. 2015;75(6):500–507. doi:10.3109/00365513.2015.1057759
14. Bratzler DW, Dellinger EP, Olsen KM, et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Am J Health Syst Pharm. 2013;70(3):195–283. doi:10.2146/ajhp120568
15. Berrios-Torres SI, Umscheid CA, Bratzler DW, et al. Centers for disease control and prevention guideline for the prevention of surgical site infection, 2017. JAMA Surg. 2017;152(8):784–791. doi:10.1001/jamasurg.2017.0904
16. Collins GS, Reitsma JB, Altman DG, et al. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. BMJ. 2015;350:g7594. doi:10.1136/bmj.g7594
17. Zhang HT, Han XK, Wang CS, et al. Diagnosis of infection after cardiovascular surgery (DICS): a study protocol for developing and validating a prediction model in prospective observational study. BMJ Open. 2021;11(9):e048310. doi:10.1136/bmjopen-2020-048310
18. American Thoracic S, Infectious Diseases Society of A. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171(4):388–416. doi:10.1164/rccm.200405-644ST
19. Kalil AC, Metersky ML, Klompas M, et al. Management of Adults With Hospital-acquired and Ventilator-associated Pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63(5):e61–e111. doi:10.1093/cid/ciw353
20. Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43(3):304–377. doi:10.1007/s00134-017-4683-6
21. Li P, Enea NS, Zuk R, et al. Performance characteristics of a high-sensitivity cardiac troponin assay using plasma and whole blood samples. Clin Biochem. 2017;50(18):1249–1252. doi:10.1016/j.clinbiochem.2017.08.002
22. Sen AC, Morrow DF, Balachandran R, et al. Postoperative Infection in developing world congenital heart surgery programs: data from the international quality improvement collaborative. Circ Cardiovasc Qual Outcomes. 2017;10(4). doi:10.1161/CIRCOUTCOMES.116.002935
23. van Buuren S, Groothuis-Oudshoorn K. Mice: multivariate imputation by chained equations in R. J Stat Softw. 2011;45(3):1–67. doi:10.18637/jss.v045.i03
24. Friedman JH, Hastie T, Tibshirani R. Regularization paths for generalized linear models via coordinate descent. J Stat Softw. 2010;33(1):1–22. doi:10.18637/jss.v033.i01
25. Alleyne CH, Hassan M, Zabramski JM. The efficacy and cost of prophylactic and perioprocedural antibiotics in patients with external ventricular drains. Neurosurgery. 2000;47(5):1124–7; discussion 27–9. doi:10.1097/00006123-200011000-00020
26. Wang D, Chen X, Wu J, et al. Development and validation of nomogram models for postoperative pneumonia in adult patients undergoing elective cardiac surgery. Front Cardiovasc Med. 2021;8:750828. doi:10.3389/fcvm.2021.750828
27. Kilic A, Ohkuma R, Grimm JC, et al. A novel score to estimate the risk of pneumonia after cardiac surgery. J Thorac Cardiovasc Surg. 2016;151(5):1415–1420. doi:10.1016/j.jtcvs.2015.12.049