Congenital disorders
Perinatal Depression: A Guide to Detection and Management in Primary Care
Abstract
Introduction: Existing guidelines for primary care clinicians (PCCs) on the detection and management of perinatal depression (PD) contain important gaps. This review aims to provide PCCs with a summary of clinically relevant evidence in the field.
Methods: A narrative literature review was conducted by searching PubMed and PsycINFO for articles published between 2010 to 2023. Guidelines, systematic reviews, clinical trials, and/or observational studies were all examined.
Results: Screening with the Edinburgh Postnatal Depression Scale or Patient Health Questionnaire-9 followed by a diagnostic evaluation for major depressive disorder in probable cases can enhance PD detection. At-risk individuals and mild to moderate PD should be referred for cognitive behavioral therapy or interpersonal psychotherapy when available. Selective serotonin reuptake inhibitors should be used for moderate to severe PD, with sertraline, escitalopram, or citalopram being preferred first. Using paroxetine or clomipramine in pregnancy, and fluoxetine or doxepin during lactation is generally not preferred. Gestational antidepressant use is associated with a small increase in risk of reduced gestational age at birth, low birth weight, and lower APGAR scores, though whether these links are causal is unclear. Sertraline and paroxetine have the lowest rate of adverse events during lactation. Consequences of untreated PD can include maternal and offspring mortality, perinatal complications, poor maternal-infant attachment, child morbidity and maltreatment, less breastfeeding, and offspring developmental problems.
Conclusions: These clinically relevant data can support the delivery of high-quality care by PCCs. Risks and benefits of PD treatments and the consequences of untreated PD should be discussed with patients to support informed decision making.
Introduction
Major depressive disorder (MDD) occurring during pregnancy and/or the first postpartum year (ie, perinatal depression [PD]) is a significant public health problem. With a prevalence of 9.2 to 17.0%,1⇓⇓–4 PD is associated with an increased risk of maternal and offspring morbidity5⇓⇓–8 and mortality,7,9⇓–11 as well as perinatal complications,12⇓⇓⇓⇓–17 impaired maternal-infant attachment,18 poorer breastfeeding practices,13,19,20 and offspring developmental problems.21⇓⇓⇓–25 It also raises depression risk in partners26,27 and a single untreated PD case can costs society $USD 97,209 (£75,728 [June 16th, 2023]) over the lifespan.28
Unfortunately, up to 69% of PD cases go undetected, and just 6 to 9% of those identified receive evidence-based treatment.29 Research suggests that primary care clinicians (PCCs) receive limited training in perinatal mental disorders, including evidence-based methods of detection.30⇓⇓–33 Two recent reviews30,31 suggest that existing guidelines for PCCs (eg, 2016 United States Preventive Service Task Force [USPSTF] recommendation statement)34 may be less clear than is ideal in describing which pharmacologic agents should be prescribed perinatally. Furthermore, PCCs generally feel that existing guidelines provide minimal information on the safety of antidepressants perinatally, which leads them to have to consult with other sources (eg, pharmacists and psychiatrists) before prescribing them to those with PD.30,31 These issues may contribute to the low uptake of guideline recommendations for PD among PCCs,30⇓–32 despite the fact that they feel more responsible for detecting and managing PD than obstetricians or pediatricians.30
To support PCCs in providing better care to individuals with PD, we conducted a narrative literature review in which the primary aim was to summarize existing evidence for detecting and managing PD with a focus on clinical relevance. A secondary aim was to provide up-to-date data on the risks of perinatal antidepressant use and the consequences of untreated PD to help guide PCCs in their ability to support informed decision making for patients.
Methods
PubMed and PsycINFO were searched for published articles between January first, 2010 to June sixth, 2023 using the following key terms: perinatal depression, postpartum depression, antenatal depression, guideline, diagnosis, psychosocial intervention, psychotropic drugs, teratogens, breastfeeding, and lactation. Guidelines, systematic reviews, controlled studies, or observational research addressing the aims of our review were included. Reference sections of included articles were also examined for any pertinent citations relevant to our review.
We first considered the work of specialist guidelines followed by systematic reviews of randomized controlled trials (RCTs) and then individuals RCTs. If unavailable, we then considered systematic reviews of observational research followed by large individual observational studies. Search for articles were performed between September 1, 2022, and June 6, 2023.
The Strength of Recommendation Taxonomy35 was used to evaluate evidence for detecting and managing PD. When possible, we described the magnitude of applicable risks (eg, risk ratio) reported among included studies to enhance their clinical relevance. A relative risk of 2 or more (Risk ≥2.0) is generally accepted as being clinically significant at the level of an individual patient.36 To ensure that our narrative literature review was conducted and reported appropriately, we followed recommendations from the Scale for the Assessment of Narrative Review Articles (SANRA),37 a quality assessment tool for narrative reviews.
Detection of Perinatal Depression
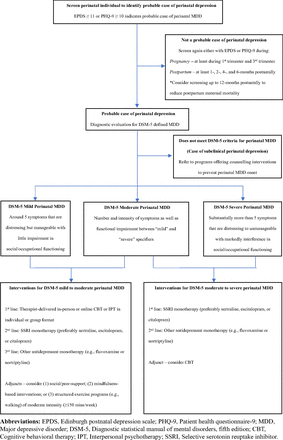
The USPSTF recommends that all perinatal individuals be screened for depression with the Edinburgh Postnatal Depression Scale38 (EPDS) followed by a diagnostic evaluation for MDD among those identified as probable cases.34 Screening programs for PD have been proven to be efficacious in reducing PD onset39 (Table 1). The EPDS is a free, 10-item self-report questionnaire that focuses on the affective and cognitive symptoms of MDD.38 It has been translated into 50 languages and only takes 5 minutes to complete.40 Although the USPSTF did not recommend a specific cutoff score,34 an EPDS score of ≥10 may suggest PD.41 However, more recent data suggest that an EPDS score of ≥11 may provide the best combination of sensitivity and specificity.42 The following EPDS ranges can be considered when assessing PD severity: none-to-minimal depression (0 to 6); mild depression (7 to 13); moderate depression (14 to 19); and severe depression (19 to 30).43 Given that PD cases commonly present with elevated levels of anxiety,44,45 EPDS items 3 to 5 (EPDS-3A Scale) can help monitor anxiety symptoms among PD cases46 and screen for a possible comorbid anxiety disorder with an EPDS-3A cutoff score of ≥5.47,48
Strength of Recommendation Taxonomy Criteria for Recommendations of Screening and Managing Perinatal Depression
The National Institute for Health and Care Excellence (NICE; UK perinatal psychiatric guidelines) and the American Academy of Pediatricians (AAP) recommend the use of the Patient Health Questionnaire-949 (PHQ-9) for assessing individuals who may have PD.41,50 A PHQ-9 score ≥ 10 seems to have a good level of sensitivity and specificity for detecting probable PD cases.51 Within USA settings, the PHQ-9 may be more practical to use than EPDS given that its generally used in primary care to screen for depression among the general population34 and could prevent PCCs from having to learn and use another depression screening tool. In addition, USA primary care sites are measured by their use of PHQ-9 for quality and reimbursement, and so may prefer to use PHQ-9 to screen for PD. Lastly, there is more supportive training and material for PHQ-9 than EPDS within USA settings. PCCs should be aware that PHQ-9 measures somatic symptoms of MDD, which can be influenced by the physiologic changes occurring in the perinatal period.40,52
PCCs should consider following the Centre of Perinatal Excellence (COPE; Australian perinatal psychiatric guidelines) recommendation of screening for pregnancy-related MDD (antenatal depression [AD]) at least during the first and third trimesters,53 which is when AD prevalence is highest.2 For postpartum MDD (PPD), PCCs can consider following the AAP recommendation of screening PPD at least at 1-, 2-, 4-, and 6-months postnatally either during well-child visits or during separate appointments.41 The Centers for Disease Control and Prevention (CDC) recently highlighted that a large proportion of maternal deaths occur between 6 to 12 months postnatally and that mental health conditions like PD are the leading cause of perinatal maternal mortality.54 With nearly 84% of these maternal deaths being preventable, it may also be beneficial for individuals to be screened for PPD up to 12-months postnatally.
Probable PD cases will require a diagnostic assessment for MDD defined by the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition55 (DSM-5). Sadness and anhedonia may not be as prominent in PD compared with MDD at other times in life.56 Furthermore, elevated levels of anxiety, restlessness/agitation, impaired concentration/decision making, and obsessional thoughts (eg, of harm coming to their infant) may be more common in PD.56⇓–58 Significant changes in weight, appetite, sleeping patterns, and energy levels will need to be further investigated as the nature of these features may be physical complaints related to the perinatal period and not necessarily somatic symptoms of MDD.40,52 Probable cases that do not meet the diagnostic threshold for MDD can be referred to evidence-based counseling interventions including cognitive behavioral therapy (CBT) and interpersonal psychotherapy (IPT), as this is recommended by the USPSTF to help prevent PD among those with subclinical depression.59,60
USPSTF also recommends evidence-based counseling interventions to at-risk perinatal individuals who present with or have combination of a history of depression, elevated anxiety levels, low economic resources, adolescent or single parenthood, domestic or intimate partner violence, or a history of significant negative life events.59,60 In those without risk factors for PD, psychological interventions seem to have minimal to no effect in preventing PPD.61
Management of Perinatal Depression
Identifying and Monitoring the Severity of Perinatal Depression
Because some treatment recommendations from guidelines are based on depression severity, it is important to also be aware of DSM-5 definitions of MDD severity: 1) Mild: Around 5 symptoms that are distressing but manageable with little impairment in social/occupational functioning; 2) Moderate: Number and intensity of symptoms as well as functional impairment between “Mild” and “Severe” specifiers; and 3) Severe: Substantially more than 5 symptoms that are distressing to unmanageable with markedly interference in social/occupational functioning.55 In addition to clinical judgment, the EPDS or PHQ-9 can aid PCCs in assessing improvements in PD severity. A 4-point reduction in EPDS62,63 or a 2 to 5-point reduction in PHQ-9 are suggestive of a clinically significant improvement in PD.64–66
Mild to Moderate Perinatal Depression
For PD of mild to moderate severity, guidelines generally recommend therapist-delivered online or in-person CBT or IPT in individual or group format as a first-line intervention,50,53,67,68 as these psychotherapies have a moderate effect in treating PD.69 Although therapist-guided online psychotherapies (especially CBT) may be effective,70 their compliance may be low and dropout rates high.70
The NICE guidelines recommend antidepressants for mild PD cases with a history of severe depression.50 However, other guidelines like those prepared by the Canadian Network for Mood and Anxiety Treatments (CANMAT; Canadian psychiatric guidelines) considers antidepressant monotherapy as a second-line intervention for mild to moderate PD67 (after therapist-delivered CBT/IPT). However, in contexts where evidence-based psychotherapies may not be easily available, PCCs should consider selective serotonin reuptake inhibitors (SSRIs) as a first-line treatment.53 In terms of specific antidepressants, CANMAT recommends either first using sertraline, escitalopram, or citalopram.67 Sertraline or citalopram use are also supported by Danish guidelines,68 and the Agency of Health care Research and Quality from the USA supports using sertraline for PPD.71 CANMAT also specifies other antidepressants like serotonin norepinephrine reuptake inhibitors (SNRIs) can be considered as third-line options for mild to moderate PD.67 Furthermore, CANMAT recommends nortriptyline (a tricyclic antidepressant [TCA]) as a third-line intervention for PPD during breastfeeding,67 given its similar efficacy to sertraline in treating PPD72 and its relatively safe lactation profile.73
Guidelines generally do not recommend gestational paroxetine68,71 or clomipramine67 use given their possible increased risk of congenital cardiac malformations.74⇓⇓⇓–78 Although not an absolute contraindication, several guidelines suggest avoiding fluoxetine among those breastfeeding,68,71 given its longer half-life (which may lead to breastmilk accumulation)71. Lastly, CANMAT does not prefer using doxepin during lactation.67,79
Referral of mild to moderate PD cases to local programs that provide social/peer-support,53,80 mindfulness-based interventions,67,81 and/or structured exercise programs with moderate intensity (≥150 minutes/week)67,82 may be helpful as adjuncts to therapist-delivered CBT/IPT and antidepressant treatment.
Moderate to Severe Perinatal Depression
Guidelines generally recommend SSRIs as first-line interventions for moderate to severe PD.53,67,68,71 Although no RCTs exist in evaluating antidepressants among depressed pregnant individuals, a recent Cochrane review indicates SSRIs are efficacious in reducing PPD symptoms.83 For specific SSRIs, only CANMAT specifically recommends sertraline, escitalopram, or citalopram as the first antidepressant for managing moderate to severe PD.67 Both NICE and COPE recommend CBT for moderate to severe PD,50,53 although COPE indicates that CBT should only be implemented after clinical response to SSRI monotherapy.53
Some individuals with moderate to severe PPD may receive treatment with brexanolone, a recently approved positive allosteric modulator of GABA type A receptors.84 One small meta-analysis of RCTs suggest brexanolone IV infusion can rapidly reduce depressive, anxious, and insomnia symptoms in PPD.85 In addition to being quite expensive,86 common adverse events include sedation, loss of consciousness, dry mouth, and hot flushes.84 Contraindications includes a history of hypersensitivity reactions to neuroactive steroids and end-stage renal failure.84 Although not a contraindication, other central nervous system depressants (eg, benzodiazepines) or other psychotropic medications with prosedative properties (eg, mirtazapine) should be avoided while receiving brexanolone as these medications may exacerbate its sedating effects.84
Risks of Perinatal Antidepressant Use
Research Examining Risks of Perinatal Antidepressant Use
At present, no RCTs have investigated the adverse effects of antidepressant use among pregnant individuals, and the findings of existing observational studies are confounded by indication (ie, the purported adverse effects of antidepressants may be due to the reason they were prescribed [eg, depression, anxiety] rather than the medications themselves). Moreover, some of these observational studies fail to use a comparison group of depressed pregnant people unexposed to antidepressants, and some fail to adjust for important confounding variables (eg, prepregnancy obesity or gestational tobacco/alcohol use).
As for research investigating the risks of antidepressant use during lactation, these are mostly composed of case reports or case series which tend to lack sufficient statistical power to detect associations. In addition, doses of antidepressants and follow-up periods are variable in these studies and most of the larger observational studies examining these links have inconsistently adjusted for confounding variables.
Congenital Malformations
First trimester SSRI may be associated with a slightly increased risk of congenital malformations,75 including those related to the heart75,76 (Table 2), though these links do not remain statistically significant when examined among pregnant individuals with mental illness.75 Two recent observational studies also suggest no association between in utero SSRI exposure and congenital malformation development (including severe cardiac malformations) after controlling for relevant confounds.87,88 As for specific SSRIs, first trimester exposure to sertraline, escitalopram, citalopram, or fluoxetine may not increase the risk of congenital malformations (including cardiovascular defects) among pregnant individuals with mental illness.75 However, 1 recent population-based study suggest that gestational fluoxetine use may elevate the risk of minor cardiac malformations after statistically adjusting for confounds.87
Potential Risks of Gestational Use of Antidepressants
First trimester use of SNRIs may not be associated with an increased risk of most congenital malformations89 except for cardiac defects.76,89 However, this relationship with cardiac defects may not remain statistically significant when examined among pregnant individuals with a clinical indication for an SNRI.89 Furthermore, first trimester exposure to venlafaxine or duloxetine does not seem to be linked to cardiac malformations among pregnant individuals with a clinical indication for these medications.89 Yet, there are some conflicting data suggesting that early in utero exposure to venlafaxine may raise cardiac malformation risk in addition to other types of birth defects after controlling for confounds.90
Limited data exist on the teratogenicity of tricyclic antidepressants (TCAs), but 1 meta-analysis suggests that first trimester use of most TCAs (except clomipramine) may not be associated with congenital cardiac malformations.76 Observational data suggest that early exposure to clomipramine in pregnancy may elevate the risk of cardiac malformations.77,78
In the absence of RCT study designs testing these links, PCCs need to be aware that it is not possible to conclude that gestational antidepressant use is causally related to congenital malformation development.
Gestational Outcomes
Gestational antidepressant use has been linked to a small risk of spontaneous abortion91 and stillbirth91 among pregnant individuals when used in the first trimester (but not the second or third).91 Sertraline, escitalopram, citalopram, and paroxetine seem to carry the lowest risk for spontaneous abortion, whereas fluoxetine and venlafaxine may pose a higher risk.91 In addition, gestational SSRI use may be associated with preeclampsia risk.92 Although SSRI use in pregnancy does not seem to be associated with development of gestational diabetes melitus (GDM), gestational use of either venlafaxine or amitriptyline may pose a small risk for GDM.93 Given that these results are not derived from RCTs, it is not possible to rule out that other confounding factors may affect the nature and magnitude of these links. Therefore, it is not possible to conclude that antidepressants are causally linked to these poor gestational outcomes.
Delivery and Neonatal Outcomes
Several meta-analyses have reported that gestational antidepressant use may be linked to poor delivery and neonatal outcomes among depressed pregnant individuals. One of these suggested gestational SSRI use may be associated with an earlier gestational age94 and lower 5-minute APGAR score.94 Gestational antidepressant use is also associated with a small increase in risk of low birth weight infants91 and preterm birth.12 Once again, lack of using RCT study designs complicate conclusions of causality among these reported associations.
Third trimester antidepressant use may slightly raise the risk of postpartum hemorrhage with risks being lower for SSRIs than SNRIs95. In addition, third trimester antidepressant exposure may be linked to the development of poor neonatal adaptation syndrome,94,96 a combination of autonomic dysfunction, neuromuscular problems, poor feeding, and/or hypoglycemia.94,97,98 It is generally mild97 and can occur in 0 to 30% of neonates exposed to antidepressants late in utero.97,98 It typically lasts 2 to 3 days and resolves with supportive care97 but can last longer if benzodiazepines are used concurrently.98 Paroxetine, fluoxetine, and venlafaxine may pose the highest risk.98
Gestational serotonergic antidepressant use may modestly increase the risk of persistent pulmonary hypertension of the newborn99,100 (PPHN), with risks higher with third trimester use.100,101 However, the absolute risk of PPHN among infants exposed to serotonergic antidepressants during gestation (0.6-3.0/1000 live births)99⇓–101 is very low and similar to infants unexposed during gestation (2.0/1000 live births).100 Furthermore, the number needed to harm is 1000 to 1615,99,100 suggesting that 1000 to 1615 pregnant individuals need to be treated with a serotonergic antidepressant to produce a single additional case of PPHN. Gestational sertraline use may have the lowest risk for PPHN followed by escitalopram, paroxetine, citalopram, and fluoxetine.100 However, 2 recent population-based studies reported no link between SSRI use either throughout pregnancy or specifically in the third trimester with PPHN development.88,102
Neurodevelopmental Outcomes
To date, gestational antidepressant use seems to have minimal to no risk for either short- or long-term neurodevelopmental and neurobehavioral outcomes in offspring.103,104 In addition, meta-analyses examining links between gestational antidepressant use and offspring neurodevelopmental disorders (eg, autism spectrum disorder [ASD]) among pregnant individuals with mental illnesses have demonstrated no consistent statistically significant associations.105⇓⇓–108 Other individual observational studies adjusting for important confounding variables have also yielded no association between gestational serotonergic antidepressant use and ASD development in offspring.109
Offspring Outcomes during Breastfeeding
Use of most antidepressants (including brexanolone) during lactation have reported a relative infant dose of ≤10% among healthy infants,110,111 which is generally the threshold used to determine whether a medication is safe to use during breastfeeding.110 A recent review evaluating multiple safety parameters suggest that sertraline, paroxetine, fluvoxamine, citalopram, and escitalopram may be the safest antidepressants to use when breastfeeding.110
One systematic review suggests the rate of adverse events among infants exposed to sertraline112 or paroxetine112 from breastmilk may be minimal (Table 3). Data on escitalopram and fluvoxamine are fewer, but their adverse event rate seems to be slightly higher.112 Fluoxetine use during lactation is associated with more adverse events in breastfed infants,112 and some suggest that this SSRI can accumulate within breastmilk given its lengthy half-life.71 Any adverse events occurring in breastfed infants often resolve spontaneously with cessation of the medication and/or breastfeeding.112 Data examining links between SSRI use during lactation and long-term developmental outcomes among breastfeed infants currently suggest no associations.112
Potential Adverse Events in Breastfed Infants Exposed to Selective Serotonin Reuptake Inhibitors During Lactation
For SNRIs, venlafaxine use during lactation has been linked to a few adverse events in breastfed infants like early neonatal weight loss and fatigue/lethargy.112,113 As for duloxetine exposure, a few breastfed infants have demonstrated dizziness, nausea, and fatigue.112,114 Among TCAs, nortriptyline may be the safest agent to use during lactation given that no cases of adverse events have been reported among 44 breastfed infants exposed to it.73 Doxepin is generally avoided during lactation as 2 infants exposed to this drug reported somnolence, difficulty breathing, poor sucking/swallowing, vomiting, and hypotonia.79
Risks of Untreated Perinatal Depression
Maternal and Offspring Mortality
When having treatment discussions with patients, the risks of untreated PD must also be considered. Recent CDC data indicate that mental health conditions like PD are a leading cause of perinatal mortality in the USA.54 In other countries, severe perinatal psychiatric disorders also increase the risk of maternal mortality11 (Table 4), particularly perinatal suicide in the context of PD.9 Such findings are concerning because 7 to 10% of perinatal individuals can experience suicidal ideation independent of mental illness.115 In addition, PD is associated with increased risks of stillbirth10 and offspring postnatal mortality.7
Consequences of Untreated Perinatal Depression
Pregnancy and Delivery Complications
AD is linked to an increased risk of gestational hypertensive disorders5 including preeclampsia6 and higher rates of preterm birth12⇓⇓⇓–16 and low birth weight neonates.14,15 Moreover, AD is associated with intrauterine growth restriction,16 smaller head growth,17 decreased body growth,17 and lower 5-minute APGAR scores.12
Postpartum Complications
PPD is associated with more offspring physical illness,7,8 hospitalization,7 and maltreatment116 as well as a decreased likelihood of exclusive breastfeeding.19,20 In addition, PPD may be linked with poorer maternal-infant attachment,18 less optimal parenting practices,117 and may elevate the risk of depression in partners26,27 and offspring.24
Offspring Developmental Problems
Offspring of individuals with PD report more socio-emotional,21,22 cognitive,22,23,25 behavioral,22 and language22 related developmental problems. In particular, PPD can increase the risk of emotional, behavioral and peer-related problems in offspring.22 Furthermore, PPD is linked to poorer general25 and performance IQ,22 memory,22 executive functioning,23 and academic achievement22 among offspring.
Conclusion
Screening with EPDS or PHQ-9 followed by a diagnostic evaluation for MDD in probable cases can enhance detection of PD. At-risk individuals and mild to moderate PD cases should be referred to therapist-delivered CBT or IPT if possible. SSRIs should be used for moderate to severe PD, with sertraline, escitalopram, or citalopram being preferred first. Generally, using paroxetine or clomipramine in pregnancy and fluoxetine or doxepin during lactation is not preferred. Antidepressant use in pregnancy and lactation has been linked to negative outcomes, but lack of RCTs testing these links limits the ability to conclude whether perinatal antidepressant use is causally related to any of the reported risks discussed in this review. Lastly, a discussion of the consequences of untreated PD can aid PCCs in their converstations with PD cases about the benefits and harms of using antidepressants. Use of these recommendations can help further enhance the high-quality care PCCs provide to those with PD, accelerating recovery with benefits for them and their families.
” data-icon-position data-hide-link-title=”0″>
Suggested algorithm for detection and management of perinatal depression within primary care.
Notes
-
This article was externally peer reviewed.
-
This is the Ahead of Print version of the article.
-
Conflict of interest: The authors have no conflicts of interest to declare.
-
Funding: RJVL is supported by the Canada Research Chairs Program.
-
To see this article online, please go to: http://jabfm.org/content/00/00/000.full.
- Received for publication February 21, 2023.
- Revision received June 17, 2023.
- Accepted for publication June 26, 2023.