Infection
Pregnant women receiving effective resistant TB treatment had ‘excellent outcomes’
September 18, 2023
2 min read
Source/Disclosures
Published by:
Disclosures:
Lachenal reports no relevant financial disclosures. Please see the study for all other authors’ relevant financial disclosures.
Key takeaways:
- Pregnant women treated with regimens including bedaquiline and/or delamanid had positive treatment outcomes.
- There were no major negative health impacts reported among the infants born.
Pregnant women who received effective multidrug-resistant/rifampicin-resistant tuberculosis treatment regimens had positive outcomes with no major negative impacts on their infants, researchers reported.
“Our work is focused on ensuring safe and efficacious treatment for all people living with drug-resistant tuberculosis (TB),” Nathalie Lachenal, pharmacovigilance officer at Médecins Sans Frontières, told Healio.
“Pregnant people living with drug-resistant tuberculosis have been systematically excluded from traditional clinical trials. Hence only extremely limited experience and scarce data are available to guide women, their families and their clinicians make informed decisions about their treatment during pregnancy
To assess , Lachenal and colleagues in the Médecins Sans Frontières pharmacovigilance unit collected data on pregnancy, birth and treatment outcomes through the endTB observational study’s Strengthening Evidence on Optimal Treatment of Multidrug-Resistant Tuberculosis (STEM-TB) cohort an extension of endTB during which patients received 9-month all-oral bedaquiline-containing regimens.
Data from STEM-TB for any patient with multidrug-resistant/rifampicin-resistant TB (MDR/RR-TB) from September 2020 through March 2023 was used for this analysis and according to the study, all individualized treatment regimens were based on National TB Program and WHO guidelines.
Lachenal explained that the current guidelines recommend doctors design individualized long regimens or a more recent recommendation a 9-month oral regimen with linezolid instead of ethionamide which has been endorsed for pregnant women without resistance to fluoroquinolones.
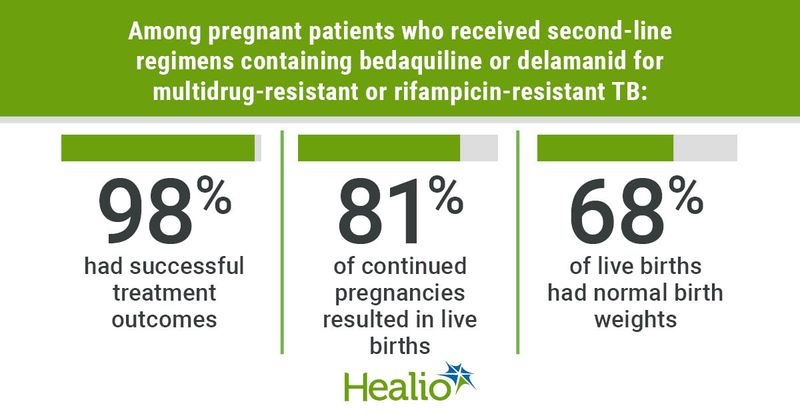
According to the study, between April 1, 2015, and March 1, 2023, 1,057 women of childbearing age received treatment with bedaquiline and/or delamanid, with 48 pregnancies from 43 women being reported to the study.
A, 73% of pregnancies began after MDR/RR-TB treatment initiation, 17% began starting treatmen, and 10% of pregnancies occurred after completion of treatment.
Among all pregnancies, 35% (17 of 48) were electively terminated, 81% (26) were live births, 13% (four) had unknown outcomes and 6% (two) ended in spontaneous abortion early on. Among the 22 live births with known birthweight, 15 infants (68%) had normal birthweight and seven (32%) had low birthweight.
Lachenal said that, overall, these results show that pregnant women treated with carefully designed long regimen including bedaquiline and/or delamanid or on 9-month regimens with bedaquiline and delamanid, “had excellent treatment outcomes.”
“Safe and efficacious treatment options including treatment with new drugs and shorter regimens are available to pregnant people living with drug-resistant tuberculosis,” Lachenal said.
“We encourage researchers to publish data on their small cohorts, include pregnant people in their clinical trials, and we hope a global pregnancy registry can emerge from the current effort to increase global knowledge and understanding of treatment during pregnancy