Blood
FDA approves new therapy that would reverse blood flow in leg to avoid amputation
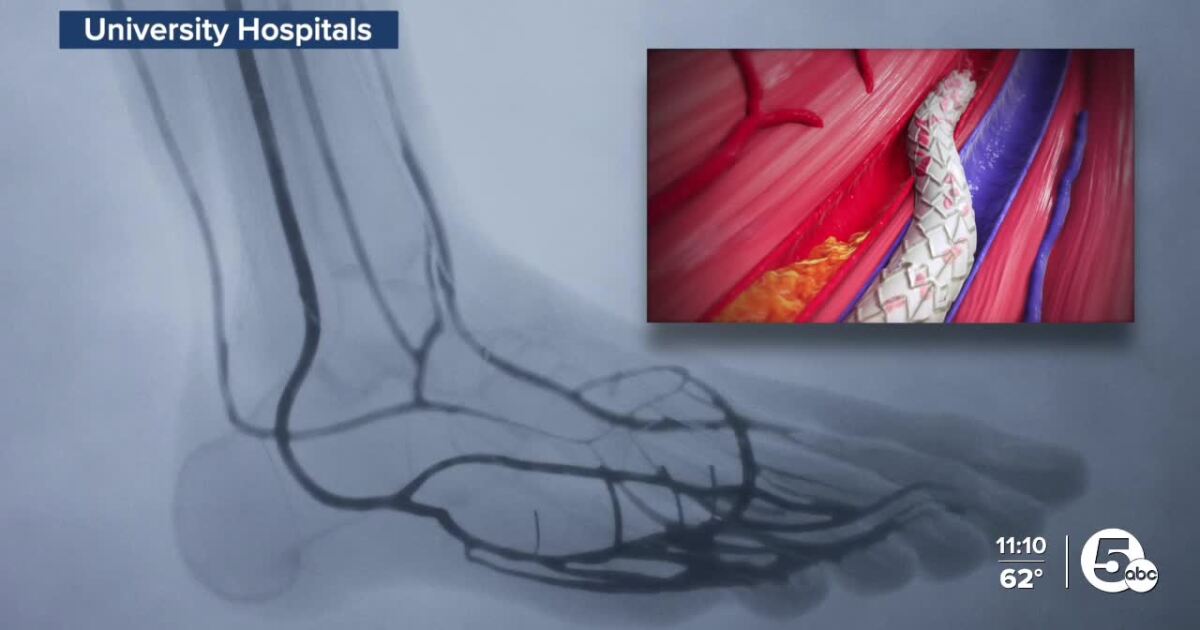
The FDA has approved LimFlow, a new therapy pioneered at University Hospitals., which gives thousands of patients hope for an alternative to leg amputation.
LimFlow therapy works by diverting blood flow from blocked arteries into the veins, making the veins become an artery, reversing the flow of blood to get oxygen in the blood to the foot.
This procedure will help thousands suffering from a vascular disease known as chronic limb-threatening ischemia, or CLTI.
Dr. Mehdi Shishehbor, the lead author and co-principal investigator of the LimFlow therapy study, spoke to News 5 about the revolutionary procedure.
“I’ve had many times patients tell me they’d rather die than lose a leg,” Shishehbor said. “I think knowing there’s hope, there’s now an option when patients have been told there’s no options available, is a big deal.”
Shishehbor said the next step is getting doctors across the country educated and up to speed to help treat patients.
“Research is hope, and this is a novel new technology,” he said. “This is technology we didn’t even know. It’s safety, it’s efficacy. That’s why research is so critical.
CLTI is the most severe form of peripheral artery disease and often occurs in patients suffering from diabetes, coronary artery disease, dialysis, obesity, high cholesterol and high blood pressure, according to a news release from LimFlow.