Infection
How siRNA Protects Mosquitoes from Viral Infections Like Zika and Dengue
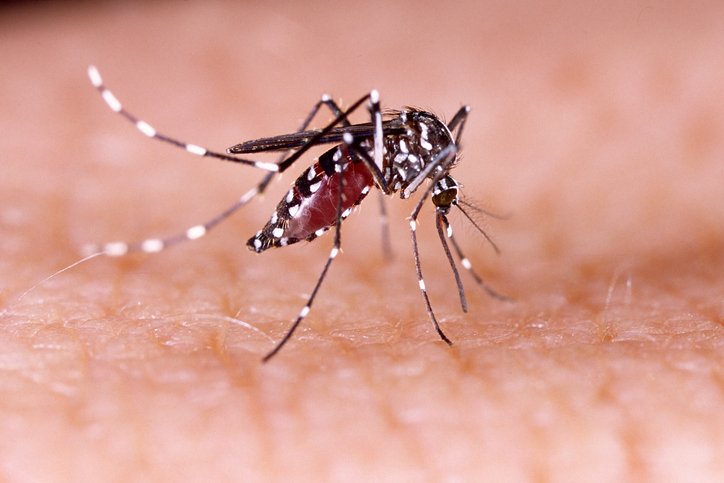
Aedes aegypti mosquitoes transmit viruses that are responsible for a significant global public health burden such as Zika, dengue, and yellow fever. Now, researchers found that an Ae. aegypti protein, Argonaute 2, has a key role—via several biological mechanisms—in keeping mosquitoes healthy and active despite these infections. These findings could lead to better methods for reducing the mosquito-to-human transmission of viruses.
The discovery represents a significant advance in understanding mosquito biology and presents a strategy that would aim to shut down Ae. aegypti mosquitoes’ defenses when they are infected by viruses—killing the mosquitoes and reducing the transmission of those viruses by Ae. aegypti to humans. Instead of making mosquitoes more resistant to the viruses, the discovery opens a possible path for making mosquitoes more susceptible and less tolerant to virus infection, which would impair their ability to transmit disease.
The research was published in Nature Communications in the paper, “Aedes aegypti Argonaute 2 controls arbovirus infection and host mortality.”
“Researchers have long wondered why Ae. aegypti mosquitoes don’t get sick when they are infected by these viruses—our findings effectively solve this mystery and suggest a potential new mosquito-based disease control strategy that merits further study,” said George Dimopoulos, PhD, professor at the Johns Hopkins Malaria Research Institute and the Bloomberg School’s department of molecular microbiology and immunology.
Ae. aegypti mosquitoes transmit arboviruses including dengue virus, yellow fever virus, Zika virus, chikungunya virus, and Mayaro virus. There are no antiviral therapies for any of these viruses. Currently, a vaccine is available for yellow fever virus. One dengue vaccine is approved by the FDA for individuals between six and 16 who have had prior dengue infection.
Ae. aegypti mosquitoes are effective vectors of arborviruses because they can sustain significant infections with these viruses without suffering costs to their overall fitness.
In the new study, researchers examined the role of Argonaute 2 (Ago2), a protein involved in the small interfering RNA (siRNA) pathway, which recognizes and destroys viral RNAs.
The researchers found that in Ae. aegypti mosquitoes lacking the Ago2 gene, the siRNA pathway is impaired, arborvirus infection becomes more severe, and the mosquitoes’ ability to transmit these viruses drops sharply—because they sicken, feed less, and often die within days.
The scientists showed that this increased mortality is caused not only by the impairment of the siRNA antiviral pathway, but also by defects in two other processes that happen to depend on Ago2: DNA repair and autophagy. Ago2-deficient mosquitoes exposed to arborviruses were left with hyperinfections, extensive DNA damage, and the accumulation of molecular waste in their dying cells.
More specifically, the authors wrote, “siRNA pathway disruption by CRISPR/Cas9-based Ago2 knockout impaired the mosquitoes’ ability to degrade arbovirus RNA leading to hyper-infection accompanied by cell lysis and tissue damage. Ago2 disruption impaired DNA repair mechanisms and the autophagy pathway by altering histone abundance.”
Apart from illuminating an important aspect of Ae. aegypti biology, the findings point to a possible new arboviral disease control strategy. This would be to engineer the mosquitoes so that arbovirus infections trigger the loss of their tolerance mechanisms, perhaps via the inhibition of Ago2. Arborvirus-carrying Ae. aegypti mosquitoes would thus die quickly, whereas the much greater number of non-arborvirus carrying Ae. aegypti should be unaffected.
“The biology of mosquito susceptibility and tolerance to infection is an interesting area of exploration for other pathogens as well,” said Dimopoulos. “For instance, mosquitoes that transmit malaria parasites could perhaps also be engineered to become sick and succumb to infection.”