Congenital disorders
Fish Reveal Caffeine and Other Common Drugs May Alter Human Facial Development
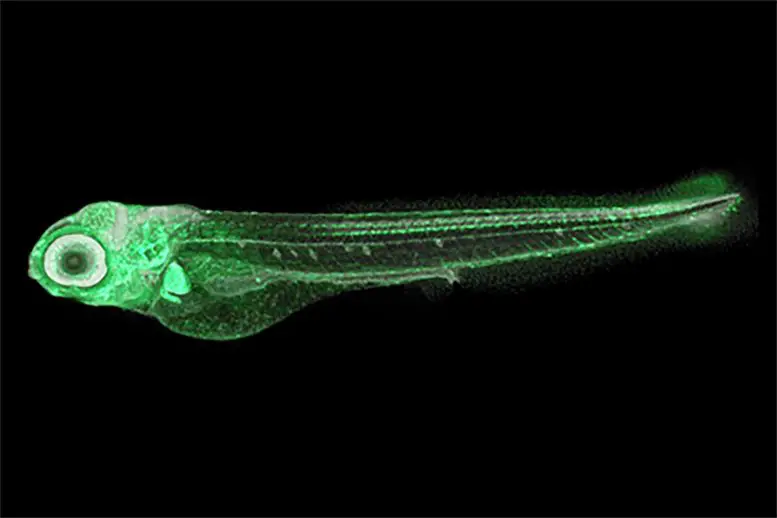
This zebrafish larva has been genetically modified so that the bone-forming cells in the face emit a green fluorescence. Normally, they are colorless and transparent, thus almost invisible at this stage. As embryos, their head and tail start to form after just 16 hours. As adults, they grow to be just 2-5 centimeters long. Credit: 2023 Liu et al.
Impact of substances on zebrafish embryos shows how the prenatal development of human facial features might also be affected.
Some substances in medicines, household items, and the environment are known to affect prenatal child development.
In a study published in Toxicological Sciences, researchers tested the effects of five drugs (including caffeine and the blood thinner warfarin) on the growth of zebrafish embryos. They found that all five had the same effect, impairing the migration of bone-forming cells which resulted in the onset of facial malformation.
Zebrafish embryos grow quickly, are transparent, and develop outside of the parent’s body, making them ideal for studying early development. A zebrafish-based system could be used to easily screen for potentially harmful substances, reducing animal testing on mammals and supporting parents-to-be when making choices for themselves and their baby.

These fluorescent images of live zebrafish embryos show the movement, assembly, and growth of cartilage-forming cells at 48, 72, and 96 hours post-fertilization. Credit: 2023 Liu et al.
Facial Differences: Causes and Prevalence
Whether from birth or through events that happen in life, many people have differences in their facial appearance. Worldwide, over one-third of all congenital anomalies relate to the development of a child’s head or facial bones — their craniofacial features — a common example being having a cleft lip and/or palate. The exact cause of craniofacial differences is not fully understood, but researchers currently think that multiple factors may be involved. This includes genetics, the gestational parent’s environment, their diet, some illnesses, and certain drugs or chemicals.
Teratogens and Testing Methods
Teratogens are substances known to disturb the growth of an embryo or fetus; for example, pregnant people are advised to avoid alcohol and nicotine. Potential teratogens are typically screened for using animals such as rodents and rabbits. However, researchers are looking for alternative methods that are quicker, cheaper, and reduce the need for testing on mammals.

These images show the development of a zebrafish’s craniofacial cartilage (via fluorescent staining) 96 hours post fertilization, comparing typical development (on the far left) with the effect of the five drugs tested. Credit: 2023 Liu et al.
Zebrafish: A Teratogen Detector
This is where zebrafish come in. These tiny, 2-5 centimeter freshwater fish grow very quickly, developing as much in a day as a human embryo would in a month. “Zebrafish embryos are transparent and grow outside the mother, so we can monitor the behavior of live cells as they develop,” said Toru Kawanishi, project assistant professor at the University of Tokyo’s Department of Biological Sciences at the time of the study. Within the past 10 years, several research projects have shown that zebrafish can effectively be used to check for teratogens. However, the exact mechanisms by which teratogens impair or alter typical embryonic development is still being investigated.
Study Insights
The team focused on a specific genetic marker for a group of cells involved in craniofacial development in both mammals and fish. In humans, these are known to become parts of the nose and jaw. “We manipulated the genome of zebrafish embryos and made bone-forming cells fluorescently visible in green. We then treated them with chemicals that are known to cause facial defects in human newborns, and tracked the trajectories of the bone-forming cells throughout embryonic stages,” explained Kawanishi.
The team tested five chemicals: valproic <span class="glossaryLink" aria-describedby="tt" data-cmtooltip="
” data-gt-translate-attributes=”[{“attribute”:”data-cmtooltip”, “format”:”html”}]”>acid (used to treat neurological and psychiatric disorders), warfarin (an anticoagulant), salicylic acid (popular in skin ointments), caffeine, and methotrexate (used in chemotherapy). They saw that, as expected, all the chemicals tested caused various degrees of craniofacial anomalies 96 hours after fertilization. However, they were surprised by the mechanism which caused this to happen and how quickly it started.
“Bone- and cartilage-forming cells in the head, called cranial neural crest cells (CNCCs), generally move a long distance from where they are first formed around the back of the neck, to their intended destinations such as the jaw or nose,” explained Kawanishi. “We were surprised that regardless of how each chemical acts on cells molecularly, impaired migration of bone-forming cells in early development was responsible for the onset of facial malformation for all the five chemicals. We could see signs of this within just 24 hours, at a point where zebrafish and mammalian embryos share very similar morphological and molecular characteristics.”
The results indicate the potential existence of a general mechanism by which teratogenic chemicals limit movement of CNCCs early on in embryos, causing the development of facial differences. The researchers extrapolate that facial differences caused by other substances might also follow the same mechanism.
“We will aim to reveal the molecular mechanism underlying the impaired cell migration, to understand why different chemicals lead to the shared defects in cell migration,” said Kawanishi.
The team proposes using this zebrafish-based system as another way to test for cross-<span class="glossaryLink" aria-describedby="tt" data-cmtooltip="
” data-gt-translate-attributes=”[{“attribute”:”data-cmtooltip”, “format”:”html”}]”>species teratogens, so that parents and medical practitioners can be made aware to limit or avoid them.
Reference: “Identification of an adverse outcome pathway (AOP) for chemical-induced craniofacial anomalies using the transgenic zebrafish model” by Shujie Liu, Toru Kawanishi, Atsuko Shimada, Naohiro Ikeda, Masayuki Yamane, Hiroyuki Takeda and Junichi Tasaki, 2 August 2023, Toxicological Sciences.
DOI: 10.1093/toxsci/kfad078