Congenital disorders
Fish uncover origins of altered human facial features
In a groundbreaking study, researchers have turned to fish to gain insights into the underlying causes of altered human facial development. Using transgenic zebrafish as a model, they identified an adverse outcome pathway (AOP) for chemical-induced craniofacial anomalies. This new research from the University of Tokyo offers new perspectives on understanding the origins of facial abnormalities in humans.
In a recent study published in Toxicological Sciences, researchers looked at how certain substances found in medicines, household items, and the environment can affect a baby’s development before birth. They tested five drugs on zebrafish embryos: caffeine and a blood thinner called warfarin. They discovered that all of these drugs had a similar effect: they interfered with the movement of cells that form bones, which led to problems in the development of the baby’s face.
Zebrafish embryos grow fast, are see-through, and develop outside their parent’s body, making them great for studying early development. Using zebrafish can help screen for harmful substances without testing on mammals, helping parents-to-be make safer choices for themselves and their baby.
Many people have differences in their facial appearance, which can be present from birth or occur due to various life events. Worldwide, more than a third of congenital anomalies involve how a child’s head and facial bones develop, known as craniofacial features.
A typical example is a cleft lip or palate. We need to understand what causes these differences entirely. However, researchers believe it’s likely a combination of factors, including genetics, the pregnant woman’s environment, their diet, some illnesses, and certain drugs or chemicals.
Teratogens are substances that can harm the growth of an embryo or fetus, and pregnant individuals are often told to avoid things like alcohol and nicotine because they can be teratogenic. Typically, researchers use animals like rodents and rabbits to test for potential teratogens. However, researchers are now searching for faster, cheaper, and more ethical ways to do this testing without using mammals.

This is where zebrafish play a vital role. These small freshwater fish undergo rapid growth, measuring just 2-5 centimeters. They can develop in a single day, which takes a human embryo a whole month to achieve.
Toru Kawanishi, project assistant professor at the University of Tokyo’s Department of Biological Sciences at the time of the study, said, “Zebrafish embryos are transparent and grow outside the mother, so we can monitor the behavior of live cells as they develop.”
In the last decade, research has demonstrated that zebrafish are helpful for identifying teratogens that harm fetal development. However, researchers are still studying how exactly teratogens disrupt average embryo growth.
The research team focused on a particular genetic marker related to cells crucial for craniofacial development in both fish and mammals. These cells eventually become parts of the nose and jaw in humans.
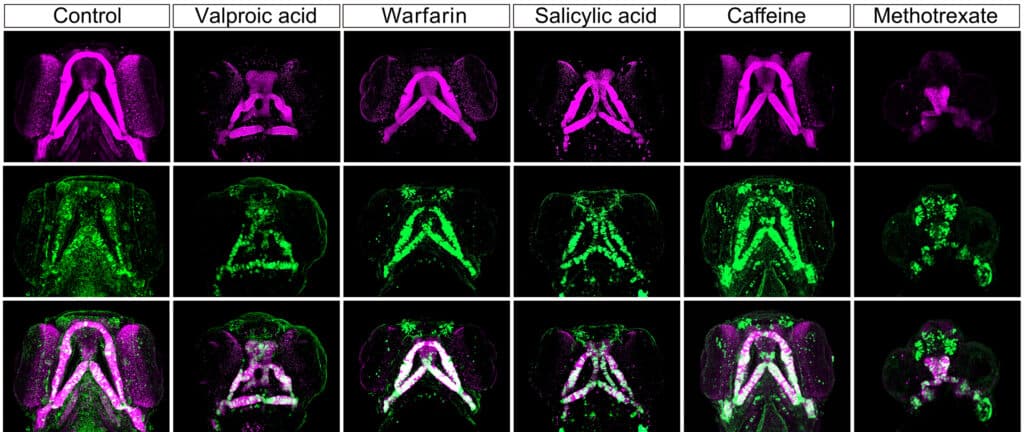
To study this, they altered the genetic makeup of zebrafish embryos, making bone-forming cells glow green. Then, they exposed these embryos to chemicals known to cause facial defects in human newborns. They carefully tracked the movements of these bone-forming cells during the early stages of development.
The researchers tested five different chemicals, including valproic acid (used for neurological and psychiatric disorders), warfarin (an anticoagulant), salicylic acid (common in skin ointments), caffeine, and methotrexate (used in chemotherapy). As expected, all these chemicals caused various levels of facial anomalies in the zebrafish embryos after 96 hours. However, what surprised the researchers was how quickly and through what mechanism this occurred.
They found that a particular type of cells in the head, called cranial neural crest cells (CNCCs), which later become bone and cartilage in the jaw and nose, usually move from their initial location around the back of the neck to their intended destinations.
The chemicals interfered with this cell movement during early development, leading to facial malformations. Remarkably, this disruption was evident within just 24 hours, a stage when zebrafish and mammalian embryos share very similar characteristics.
The study’s findings suggest that teratogenic chemicals may share a common way of limiting the movement of cranial neural crest cells (CNCCs) in early embryos, leading to facial differences.
The researchers plan to investigate the molecular details of this impaired cell migration to understand why different chemicals produce similar defects. They propose using the zebrafish model to test for potentially harmful substances that affect multiple species. This can help inform parents and healthcare providers about what to avoid during pregnancy.
Journal reference:
- Shujie Liu, Toru Kawanishi, et al., Identification of an adverse outcome pathway (AOP) for chemical-induced craniofacial anomalies using the transgenic zebrafish model. Toxicological Sciences. DOI: 10.1093/toxsci/kfad078.