Infection
Immunoglobulin prevents infections associated with anti-BCMA therapy for multiple myeloma
October 02, 2023
3 min read
Source/Disclosures
Published by:
Disclosures:
Polish National Agency for Academic Exchange supported the study. Lancman reports advisory board roles with Janssen and Pfizer. Please see the study for all other authors’ relevant financial disclosures. Garfall reports funding and/or consulting fees from Bristol Myers Squibb, CRISPR Therapeutics, GSK, Janssen, Legend Bio, Novartis, and Tmunnity Therapeutics. Stadtmauer reports consulting fees from AbbVie, Amgen, Bristol Myers Squibb, Janssen and Takeda.
Key takeaways:
- IV immunoglobulin supplementation reduced the risk for developing severe infections among those receiving anti-BCMA therapy.
- All treatment responders experienced severe hypogammaglobulinemia.
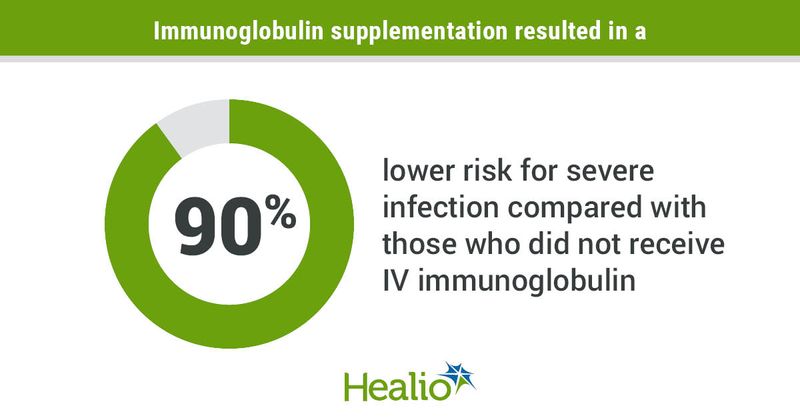
Prophylactic IV immunoglobulin supplementation reduced the risk for severe infections by 90% among adults receiving a B-cell maturation antigen-directed bispecific antibody for treatment of multiple myeloma, study results showed.
The findings — published in Blood Cancer Discovery — appear to support International Myeloma Working Group (IMWG) guidelines recommending routine use of IV immunoglobulin supplementation for all patients receiving B-cell maturation antigen (BCMA)-directed bispecifics and who have IgG levels < 500 mg/dL, according to researchers.
“All patients responding to these anti-BCMA bispecifics develop profound hypogammaglobulinemia, which seems to be driving many of the serious infections,” Guido Lancman, MD, MSc, clinical associate at Princess Margaret Cancer Centre/University Health Network and adjunct assistant professor at University of Toronto, told Healio.

Guido Lancman
“Until now there hasn’t been any evidence to support [the IMWG] recommendation and uptake has been far from 100%, even at large academic centers,” he added. “We hope this study will raise awareness about this important issue so that patients can receive optimal infection prophylaxis and continue to benefit from these highly effective therapies.
Background
BCMA-directed bispecifics have shown “impressive efficacy” in clinical trials for treatment of multiple myeloma, but this has been accompanied by high rates of serious infections, Lancman said. This will become an increasingly important issue as bispecifics work their way into earlier lines of therapy for the disease, thereby increasing recipients’ exposure to infections, he added.
“We wanted to understand what was causing these infections and see if there was anything that could mitigate this significant risk,” Lancman said.
Methodology
Lancman and colleagues conducted a retrospective analysis to characterize the risk for infection and impact of infection prophylaxis associated with the use of anti-BCMA bispecific antibodies in adults with multiple myeloma.
The analysis included 37 patients (mean age, 66 years; range, 41-85; 62% women; 46% white; 22% Black; 16% Hispanic; 8% Asian) treated with BCMA-targeted bispecifics during one of four clinical trials at Mount Sinai Hospital in New York between Jan. 1, 2019, and June 30, 2022.
Rate of grade 3 through grade 5 infections served as the study’s primary endpoint.
Key findings
Among 37 patients, 15 (41%) experienced a severe grade 3 through grade 5 infection, including two infection-related deaths among treatment-responders in deep remission.
Over a combined follow-up of 424 months, investigators reported 118 total infections among the study group, with 26 severe infections. Eighty-four percent of reported infections occurred during disease remission.
All 26 patients who responded to anti-BCMA bispecific antibodies experienced severe hypogammaglobulinemia — defined as immunoglobulin levels below 200 mg/dL — throughout the treatment period. Ninety-two percent of treatment responders received IV immunoglobin supplementation during the study treatment period.
Researchers observed a 90% lower risk for grade 3 through grade 5 infections during IV immunoglobulin supplementation compared with when study participants did not receive prophylactic supplementation (incidence rate ratio = 0.1; 95% CI, 0.01-0.8).
Researchers identified several study limitations, including its retrospective design, small sample size and lack of randomization for the administration of IV immunoglobulin.
Clinical implications
Whether immunoglobulin supplementation should be provided prophylactically to all patients receiving BCMA-directed bispecifics is a question most properly answered by a larger, prospective study, according to Lancman.
“However, in the absence of a randomized clinical trial and with many patients now receiving these therapies as standard of care, we need to act on the data and experience we have accumulated to date,” Lancman told Healio. “Our study supports the IMWG recommendation that all patients receiving anti-BCMA bispecifics should receive immunoglobulin replacement as primary prophylaxis.”
Lancman coupled this conclusion with a note of caution.
“As these bispecifics get moved to earlier lines of therapy — where the expected duration of response may be longer — it won’t be feasible to provide immunoglobulin replacement to these large numbers of patients for long periods of time,” he added. “We really need to explore fixed-duration studies of these bispecifics to allow the immune system the opportunity to recover.”
The results underscore the need for clinicians who deploy these therapies to remain vigilant about infection prophylaxis and properly warn patients about the risks associated with the use of BCMA-directed bispecifics, according to Alfred L. Garfall, MD, and Edward A. Stadtmauer, MD, from Abramson Cancer Center, Perelman School of Medicine at the University of Pennsylvania.
“More specifically, these results support universal use of [immunoglobulin replacement therapy] in relapsed/refractory multiple myeloma patients receiving anti-BCMA [bispecific antibody] therapy,” they wrote in an accompanying editorial. “Though such a small, retrospective study would not ordinarily be the basis for clinical practice recommendations, these results affirm the impressions of clinicians with experience using these agents in clinical trials as reflected in two recent consensus statements that recommend universal use of [immunoglobulin replacement therapy] in these patients.”
References:
For more information:
Guido Lancman, MD, MSc, can be reached at Princess Margaret Cancer Centre, University Health Network, 700 University Ave., 6th Floor 6-604, Toronto, ON, Canada M5G 1X6; email: guido.lancman@uhn.ca.