Cardiovascular
Midlife cardiovascular health factors as predictors of retirement age, work-loss years, and years spent in retirement among older businessmen
Abstract
Cardiovascular disease (CVD) is one of the leading causes of premature retirement. However, the relationship between CVD risk factors and workforce participation is not well known. We studied the relationship between midlife CVD risk, age at retirement, work-loss years, and survival in retirement. Middle-aged Finnish men (initial n = 3490, mean age = 47.8 years) were assessed for CVD risk factors and general health in the 1970s. They worked as business executives and provided information on their retirement status in the year 2000. Survival was followed up to the 9th decade of life with a follow-up of up to 44 years. Work-loss years were calculated as death or retirement occurring at age ≤ 65 years. Smoking, body mass index, and alcohol use were used as covariates, excluding models of CVD risk, which were adjusted for alcohol use only. Higher risk of 10-year fatal CVD was associated with 0.32 more years (relative risk < 1 vs. 1, covariate-adjusted β = 0.32, 95% CI = 0.13, 0.53) of work-loss. Higher risk of 5-year incident (covariate-adjusted time-constant HR = 1.32, 95% CI = 1.19, 1.47) and 10-year fatal (covariate-adjusted time-dependent HR = 1.55, 95% CI = 1.30, 1.85) CVD in midlife were associated with fewer years spent in retirement. Poorer self-rated health and physical fitness and higher levels of triglycerides were associated with increased hazard of earlier retirement, more work-loss years, and fewer years spent in retirement. Poorer health and greater midlife CVD risk may be associated with earlier exit from the workforce and fewer years spent in retirement. Management of CVD risk in midlife may support people to work longer.
Introduction
Cardiovascular disease (CVD), a leading cause of premature mortality worldwide1, is associated with poorer workforce participation2 among older employees by increasing the risk of transitioning into disability pension3,4 and exiting the labour force early5. It bears high costs to societies, amounting to a quarter of health-related productivity losses in the European Union, with estimates of 54 billion in the year 20196. Whether CVD risk factors, including elevated blood pressure, serum lipids, and blood glucose among others, would also associate with workforce participation among individuals of working age, is poorly understood7,8. Understanding this association is becoming increasingly important as more individuals are expected to work longer in the future, regardless of coexisting chronic illnesses.
A recent meta-analysis highlighted the need to consider prior health when studying the relationship between retirement timing and mortality9. This can only be examined with follow-up studies started at earlier stages of the life course, with subjectively and objectively measured data on health at working age and in retirement, as well as data on mortality. There are few previous studies that have investigated the association between prior health and survival in retirement over longer periods of time. Years spent in retirement is a way of describing this phenomenon and it can be calculated for cohorts with mortality follow-up until very old age.
Our aim was to increase the understanding of midlife CVD risk factors and general health as predictors for pre-retirement workforce participation and post-retirement survival; the former measured through age at retirement and calculation of work-loss years and the latter represented as survival in retirement. The rationale for including work-loss years was that it is uncertain whether previous findings are influenced by mortality before retirement. To study this, we assessed CVD risk factors and aspects of general health among Finnish male executives in midlife (mean age 47.8 years) and obtained information on their retirement status in the year 2000 and by that time the youngest participants had reached the age of 66 years. Thereafter, assessment of register-based all-cause mortality continued until the 9th decade in the year 2018. We hypothesized that greater CVD risk and poorer general health in midlife would associate with premature retirement and with poorer survival in retirement.
Methods
Study population
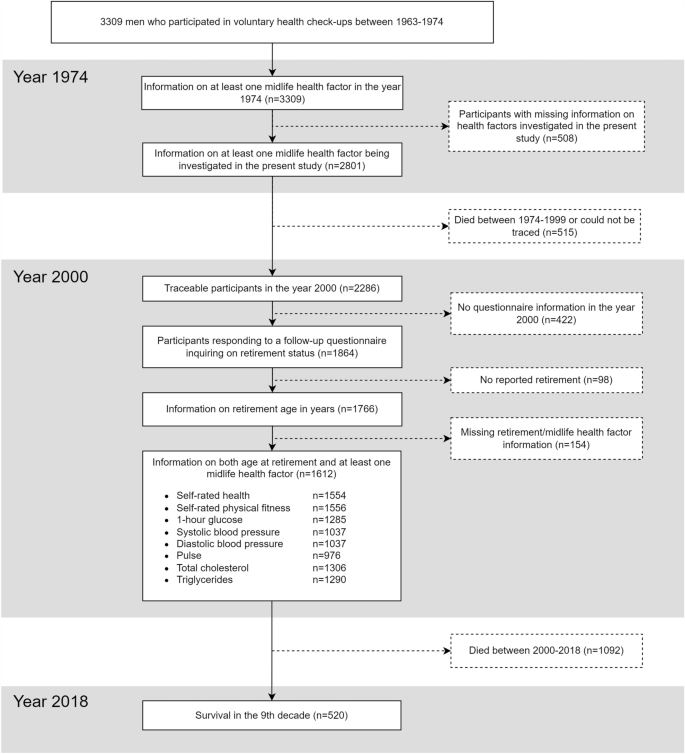
The Helsinki Businessmen Study is a longitudinal study of 3,490 initially healthy men who were born between 1919 and 193410. The present sample (n = 3,309) consists of participants who could be traced using personal identification numbers assigned to all Finnish residents in the 1970s. Figure 1 presents a flowchart of the study population. The men (mean age 47.8 years in the year 1974) participated in voluntary health check-ups covering a range of cardiovascular health factors at the Finnish Institute of Occupational Health. This information was then used to calculate estimates of the participants’ 5-year risk of incident CVD11 and 10-year risk of incident fatal CVD12. Information on retirement was obtained when the youngest participants had reached the age of 66 years in the year 2000; out of 2,286 traceable participants, 1,864 returned the questionnaire. Full retirement data were available for 1,766 participants, of whom 1,612 also had information on midlife cardiovascular health factors. The participants’ deaths were tracked from The Digital and Population data services Agency’s Population Information System between the years 1974 and 2018.
Flowchart over the 3,309 men and their inclusion in the present study. Solid lines represent groups relevant to the present study.
Cardiovascular health factors in 1974–1975
Midlife cardiovascular health factors, measured in the 1970s, included clinical and laboratory data10, such as blood pressure (BP), heart rate, fasting total cholesterol (TC)13, triglyceride levels (TG), and 1 h blood glucose levels after an oral glucose tolerance test (1 h-OGTT load)14. Participants’ global self-rated health (SRH) and self-rated physical fitness (SRF) were assessed with the question, “What do you think about your present state of health/physical fitness; is it ‘very good’, ‘fairly good’, ‘average’, ‘fairly poor’ or ‘very poor’?” SRH categories ‘fairly poor’ and ‘very poor’ were combined into ‘poor’ due to the small number of men who perceived their SRH as ‘very poor’.
We estimated the participants’ 5-year risk of incident CVD11 using a tool, that was developed among European and U.S. men aged 40–59 years free from coronary heart disease. The risk score11 uses information on age, systolic BP, serum TC, smoking status and body mass index (BMI). We also calculated the participants’ 10-year composite risk score of fatal CVD in midlife and old age using the European ‘low risk’ SCORE-chart (Finnish)12. The chart gives estimates of the relative risk of fatal CVD events in a 10-year period. SCORE has been externally validated15 and uses sex, smoking status, systolic BP, and serum TC to estimate CVD risk.
Covariates in the year 1974
In 1974, height and weight measurements were used to calculate BMI (kg/m2). Smoking status, based on self-reported data, was categorized as current smokers, ex-smokers, and never smokers. Similarly, self-reported weekly alcohol consumption in grams of pure alcohol was recorded.
Retirement age and time spent in retirement evaluated in 2018
In 2000, participants reported their retirement status and age at retirement16. We excluded participants reporting no retirement (n = 98) and analysed data from 1766 men. Time spent in retirement was calculated in years from the onset of retirement until death or the end of follow-up in the year 2018. Approximately two thirds (n = 1,999, 67.9%) of the sample had died by 2018, and we observed 43,086 person-years of mortality follow-up from the onset of retirement.
Working years lost due to death or early retirement
To study whether the inclusion of participants who died before retiring would affect our results, we calculated work-loss years17 as death or retirement occurring before the general age limit for old-age pensions. We used 65 years as the limit for old age pensions17, which in this cohort was also the most common age to retire (n = 250, 14.2%). The respective observations in person-years were 4,804 and 24,627, for deceased and those retiring younger than at age 65 years.
Statistical methods
We present continuous variables as means and standard deviations and used one-way analyses of variance and Kruskall-Wallis tests for group central tendency statistics. Categorical data are presented as proportions. Covariate-adjusted Cox proportional hazard regression models and models extended for time-dependent effects were used to estimate hazard ratios (HRs) and 95% confidence intervals. For complex associations figures of HRs are shown. When the proportionality of hazards assumption is not met for covariate Z, the Cox- regression model can be extended to handle time-dependent effects by adding an effect of covariate interaction with a function of follow-up time. Thus, in our models the hazard function, hi(t), for participant i (i = 1, …, N) was specified as:
where h0(t) is the baseline hazard function, zi is the covariate value with a time-invariant and time-dependent effects γ0 and γt, respectively, g(t) is a suitable function of time, t, and (beta) is the vector of regression coefficients for other time-invariant fixed-effects for other covariates in vector xi. Notably, the effect of covariate Z is partitioned into two components: the time-invariant effect of the predictor and a time-varying quantifying dynamically the difference to the time-invariant effect over the follow-up. The function of time can take various functional forms, and we have indicated the selected function for each predictor in the tables for time-dependent effects. To get an idea of the total impact of covariate Z, the time-varying and time-invariant effects must be interpreted together, the HR estimates themselves are difficult to digest, and it is more informative to present the hazard ratio as a figure. In absence of time-dependent effects, the model Eq. (1) becomes the conventional proportional hazards Cox regression model.
As model validation, we assessed time-dependency in model effects using plots and tests based on scaled Schoenfeld-residuals, functional form of model effects based on martingale residuals, and impact from outliers based on dfbeta-statistics. Extended model effects (non-linear and time-dependent effects) are shown using predicted hazard plots. We adjusted our models with smoking, alcohol consumption, and continuous BMI in midlife. As some of these variables were used in estimating CVD risk, analyses of CVD risk were adjusted for alcohol consumption only. Supplementary Tables 1–2 show information about support for model assumptions and count of participants at risk in our analysis regarding age at retirement. Supplementary Tables 3–4 show the respective information in our analysis on survival in retirement. We normalized work-loss data by performing a log (base 10) transformation and back-transformed the point estimates. For the models we report hazard ratios or regression coefficients together with their 95% confidence intervals. The analyses were carried out using R software18 (version 4.2.1) with packages survival19 and extrafont20, and linear regression models in Stata (Release 17. College Station, TX: StataCorp LLC).
Ethical approval
The research protocol of the follow-up study of HBS has been approved by the Ethics Committee of the Department of Medicine, University of Helsinki. The study adheres with the principles stated in the Declaration of Helsinki.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Results
Pre-retirement workforce participation: age at retirement and work-loss years
The men had retired at the mean age of 61.2 years (SD = 4.4 years). Table 1 shows that men in the lowest quintile of retirement age (< 58 years) had had poorer SRH and SRF in midlife and lower mean risk of 5- and 10-year estimated risk of non-fatal/fatal CVD than those in the highest quintile retiring at age ≥ 65 years. In addition, 441 men had died before the statutory retirement age of 65 years. The combined work-loss through retiring or dying before age 65 years was 4.8 years (SD = 4.3 years; range = 0–38 years).
Post-retirement survival
After the men had retired, their mean survival time was 24.4 years (SD = 6.6 years; range 3–54 years). The lexis diagram indicated that survival in retirement was relatively evenly distributed (Supplementary Fig. 1). Mortality increased during the study period according to the degree of estimated risk of CVD in midlife; in the year 2018 fewer than 1 out of 10 had survived in the groups with the highest risk, whereas slightly less than half in the low-risk groups were alive in the year 2018 (Supplementary Table 5).
Health in midlife and sample selection
We report findings on men who had been measured for CVD health factors in midlife and who provided information on retirement age in a questionnaire (Fig. 1). Participants who had been assessed for CVD health factors in midlife but who were not included in the present study because of non-respondence to the questionnaire (n = 557) or death (n = 889) had been less healthy in most considered health factors in midlife (Supplementary Table 6).
Self-rated health and physical fitness in midlife as determinants of pre-retirement workforce participation
Poorer SRH (average or poor vs. very good) and SRF (very good vs. fair) were associated with higher hazards of retiring at a younger age, of which poorer SRH (poor vs. very good, covariate-adjusted HR = 1.63, 95% CI = 1.14, 2.33) and SRF (very good vs. fair, covariate-adjusted HR = 0.71, 95% CI = 0.52, 0.94) remained statistically significant after adjusting for smoking, alcohol consumption, and BMI in midlife (Table 2). Similarly, men with poor SRH (poor vs. very good) had 0.32 years more work-loss (95% CI = 0.13, 0.52) in analyses adjusted with smoking, BMI, and alcohol consumption in midlife (Table 3). Poor SRF (very poor vs. fair) was initially associated with more work-loss (crude β = 0.45, 95% CI = 0.14, 0.66), but this association attenuated following adjustment for covariates (covariate-adjusted β = 0.38, 95% CI = − 0.29, 0.69).
Laboratory and clinical CVD risk factors in midlife as determinants of pre-retirement workforce participation
Some but not all individual CVD risk factors showed associations with earlier retirement or more work-loss years, with many associations becoming statistically non-significant after covariate adjustment (Tables 2 and 3). Systolic BP had a curvilinear association to retirement hazard, suggesting that those in either low or high end of the range of observed readings had a lower hazard of retiring than those with readings in the mid-range (Fig. 2A). The association was weak, and it did not change substantially after covariate adjustment, which also reduced the sample size. Similarly, one-unit increases in systolic BP and diastolic BP showed initial positive associations of more work-loss years, but these associations attenuated after adjustment for smoking, BMI, and alcohol consumption (Table 3). Time-dependent hazards of unit-higher triglyceride levels showed an elevated hazard rate (HR(t) ~ 2.2) of earlier retirement, which then levelled off by age 60 years (Table 2 and Fig. 2B). For each 1 mmol/l increase in 1 h-OGTT (covariate-adjusted β = 0.01, 95% CI = 0.00, 0.03) and triglycerides (covariate-adjusted β = 0.05, 95% CI = 0.01, 0.09), the men were more likely to have more work-loss years (Table 3).

Predicted time-dependent hazard ratios for systolic blood pressure (panel A, top) and triglyceride concentration (panel B, bottom) assessed in midlife. The curves show the time-varying HR (solid dark grey line) of retirement age in years, and reference lines for HR estimated under the proportional hazards assumption (dashed grey line) and HR for strictly equal hazards (grey, dotted line).
Composite CVD risk estimates in midlife as determinants of pre-retirement workforce participation
The associations between midlife CVD risk and retirement and work-loss years were conflicting; while higher risk of CVD was associated with retiring at an older age, there was evidence suggesting that those with high risk were more likely to have more years of work-loss (Tables 2 and 3). Higher 5- and 10-year CVD risk scores in midlife were associated with a lower covariate-adjusted hazard of retirement (Table 2). Respectively for work-loss years, each increase of one standard deviation in the 5-year risk of incident CVD was not associated with work-loss years. The association between 10-year fatal CVD risk estimates and work loss showed that individuals with a 10-year fatal CVD risk estimate of 1 or higher vs. less than 1 relative risk had 0.32 more work-loss years (covariate-adjusted β = 0.32, 95% CI = 0.13, 0.53).
Health in midlife as a determinant of post-retirement survival
Poorer SRH (average or poor vs. very good) and SRF (poor or very poor vs. fair) were associated with fewer years spent in retirement, of which poor SRH (poor vs. very good, covariate-adjusted HR = 2.05, 95% CI = 1.37, 3.07) and SRF (poor vs. fair, covariate-adjusted HR = 1.26, 95% CI = 1.20, 1.55) remained after adjustment for smoking, alcohol consumption, and BMI in midlife (Table 4). Per each 1 mmol/l higher total cholesterol (covariate-adjusted HR = 1.08, 95% CI = 1.01, 1.15) and triglycerides (covariate-adjusted HR = 1.08, 95% CI = 1.01, 1.15) the men were more likely to spend fewer years on retirement (Table 4). Higher systolic BP, diastolic BP, and midlife cardiovascular risk scores showed age-adjusted time-dependent hazard of spending years in retirement (Table 4 and Fig. 3). In panel A, unit-higher midlife systolic BP was associated with an almost linearly increasing hazard from age 75 years to HR(t) ~ 1.015 at age 97. In panel B, unit-higher midlife diastolic BP showed an exponential rate of growth in the time-dependent hazard rate of fewer years spent in retirement from age 67 years with a peak of HR(t) ~ 1.05 at age 97. In panel C, one standard deviation higher estimated 5-year incident risk of cardiovascular disease in midlife showed curvilinear time-dependent elevated hazard rates peaking at approximately age 85 years (HR(t) ~ 1.3)., which levelled off so that the hazard rate remained indicative of elevated hazard for higher risk scores. In panel D, unit-higher 10-year risk of cardiovascular disease showed a linear increase from age 82 years to HR(t) ~ 1.4 at age 97.

Predicted time-dependent hazard ratios for systolic blood pressure (panel A, top), diastolic blood pressure (panel B), 5-year risk of incident CVD (panel C), and 10-year risk of fatal CVD (panel D, bottom). The curves show the time-varying HR (solid dark grey line) of survival in years, and reference lines for HR estimated under the proportional hazards assumption (dashed grey line) and HR for strictly equal hazards (grey, dotted line).
Discussion
Cardiovascular disease (CVD) is a leading cause of disability pension and premature exit from the workforce, significantly affecting work ability and labour force participation2,5. Despite its impact, limited research has focused on studying CVD risk factors or composite CVD risk estimates in populations without pre-existing CVD. Our study, based on business executives followed for up to 44 years, found a time-dependent relationship between higher triglyceride levels in midlife and earlier retirement, independent of lifestyle-related factors including smoking, alcohol use, and BMI in midlife. Furthermore, although men with higher CVD risk in midlife were more likely to retire at an older age, we found evidence to support that they also experienced more work-loss years, i.e., to retire or die before age 65 years. After the participants had retired, those who had had higher CVD risk in their midlife also had higher mortality risk in retirement. Poorer self-rated health and physical fitness predicted earlier retirement, more work-loss years, and poorer survival in retirement.
Interpretation in light of known literature
We were able to expand the previous scarce literature on CVD risk and retirement, where both early and on-time retirees displayed similar CVD risk factors7, by providing evidence of higher triglyceride levels associating with increased hazard of earlier retirement. While this association was independent of lifestyle factors including alcohol consumption, higher triglyceride levels could still reflect overall poor health, stress, or other factors that predispose to early retirement. Poorer self-rated health, a known predictor of transitioning into disability pensions21,22,23, was also associated with increased hazard of earlier retirement in our study, thus corroborating previous findings. Individuals who self-rate their health as favourable may exhibit improved work ability and the absence of conditions which may compromise their ability to work21,22,23. However, our results suggest that CVD risk may not be a major determinant of earlier retirement overall, as most other CVD risk factors were unrelated to hazard of earlier retirement. CVD risk alone may not be sufficient to generate symptoms with significant impact work ability. Moreover, men at higher risk of developing CVD in midlife were not at increased hazard of retiring early; in fact, they showed a decreased hazard. Considering that the men underwent clinical examinations in the Finnish Institute of Occupational Health and that by old age the difference in CVD risk by retirement age appeared to have levelled off, it is possible that men with high CVD risk received interventions to mitigate their high risk.
Considering these findings and the fact that one can exit the workforce also through premature mortality, we found parallel evidence to support CVD risk factors as determinants of more work-loss years, where participants retiring or dying prematurely (younger than age 65 years) were considered. We could expand the previous literature on estimated productivity losses due to medical conditions, e.g. hypertension24,25, heterozygous familial hypercholesterolemia26, and diabetes27,28 to include CVD risk factors and estimated CVD risk by showing evidence that higher CVD risk, levels of 1 h-OGTT, and serum triglycerides in midlife were associated with more work-loss years. However, as with retirement age, most other CVD risk factors were unrelated to work-loss, and findings on CVD risk and work-loss years were less consistent.
Our follow-up extended to the 9th decade, enabling us to describe survival among the study participants and provide valuable information on the impact of CVD risk factors in old age. While CVD risk factors are known predictors of overall survival13,29,30, a meta-analysis on the association between retirement age and survival9 highlighted the significance of prior health in their analysis. Our findings contribute to this gap by suggesting that midlife CVD health factors not only potentially impact productivity losses but also contribute to fewer years in retirement. We found that men with higher CVD risk factors, higher estimated risk of 5-year incident or 10-year fatal CVD, and poorer self-rated health and fitness, had a higher hazard for all-cause mortality in retirement, regardless of smoking, alcohol consumption, and BMI in midlife. Some of these associations also showed time-dependent hazards9,13,17,24,25,26,27,28,29,30.
Importance of the results
These findings highlight the impact of CVD risk on the work-life trajectory of individuals and emphasize the importance of early identification and management of modifiable risk factors to reduce CVD-related morbidity and mortality. First, although CVD is a leading cause of premature retirement, we found no evidence to support CVD risk factors as major determinants of earlier retirement, except for triglyceride levels, self-rated health, and physical fitness. However, when premature mortality before age 65 years was also considered in combination with earlier retirement in work-loss years, greater overall CVD risk also emerged as a potential factor in productivity losses. Second, our results suggest that potential productivity losses related to higher CVD risk may also occur in healthy individuals, as only a small proportion of our study participants had CVD or diabetes in midlife. Therefore, efficient management of CVD risk may prevent potential productivity losses even among healthy individuals. Third, our study supports the notion that both general health and CVD risk factors are critical factors to consider when examining retirement outcomes and survival in general.
Strengths and limitations
We examined the association between CVD risk factors, retirement age, work-loss years, and mortality in older male executives. The study followed the men for up to 44 years, until the 9th decade of life, and used national register-linkage to gather data on mortality. The sample was subject to selective survival and loss to follow-up, leaving out participants who had poorer general and CVD health in midlife, thus potentially undermining our results regarding retirement age and survival in retirement. Given that CVD is one of the leading causes of premature exit from the workforce2,5, we believe that participants at high risk of CVD but who did not survive to retirement age may have also been more likely to retire early due to CVD-related health problems. The study population consisted of Caucasian men from higher socioeconomic groups10, which provides internal validity but significantly limits generalizability to other socioeconomic groups, women, or individuals with different retirement policies. The men worked as executives and entrepreneurs who did not have fixed retirement ages. Laboratory CVD risk factors were measured in the 1970s using methods from that time, which may be outdated, but have been shown to correlate with clinical outcomes in later studies from this cohort14. Due to the extended time span between the assessment of CVD risk factors and retirement, participants’ cardiovascular risk levels may have undergone changes or interventions, such as pharmacological treatments. Our definition of work-loss years has potential caveats as the retirement characteristics were self-reported. Nonetheless, we used a cut-off at 65 years, which has been used by others17.
Future recommendations
Efforts aimed at early identification and management of CVD risk factors may not only enhance the productivity of individuals during their working years but also contribute to a longer and healthier retirement period. To achieve this, clinicians should prioritize assessing and addressing CVD risk factors in midlife to reduce morbidity and mortality. Targeted interventions should be implemented for individuals with poorer self-rated health, while comprehensive retirement planning should incorporate cardiovascular health considerations to promote a longer and healthier retirement period. Further investigation is warranted to explore the underlying mechanisms behind the association between higher triglyceride levels and the time-dependent hazard of early retirement, as the precise mechanisms remain unclear.
Conclusions
This study found evidence that CVD risk factors can serve as predictors of both pre-retirement workforce participation and post-retirement survival. Although high midlife CVD risk was not a consistent predictor of earlier retirement, high risk may contribute to premature mortality, potentially leading to poorer pre-retirement workforce participation. Of considered CVD risk factors, only high midlife triglyceride levels were associated with the hazard to retire earlier. Midlife CVD health factors and composite risk estimates were linked to a higher hazard of mortality in retirement. Those with poorer self-rated health or fitness were at higher hazard to retire earlier, more likely to have more work-loss years, and at higher hazard for mortality in retirement, rendering them potential targets for interventions. The effective management of CVD risk factors may enhance the productivity of working-aged individuals and the survival of retirees.
Data availability
The datasets used and/or analysed during the current study available from Timo E. Strandberg on reasonable request.
References
-
Burden, G. et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: Update from the GBD 2019 study. J. Am. Coll. Cardiol. 76(25), 2982–3021 (2020).
Google Scholar
-
Bin Sayeed, M. S., Joshy, G., Paige, E., Banks, E. & Korda, R. Cardiovascular disease subtypes, physical disability and workforce participation: A cross-sectional study of 163,562 middle-aged Australians. Plos One 16(4), e0249738 (2021).
Google Scholar
-
Oude Hengel, K., Robroek, S. J. W., Eekhout, I., Van Der Beek, A. J. & Burdorf, A. Educational inequalities in the impact of chronic diseases on exit from paid employment among older workers: A 7-year prospective study in the Netherlands. Occup. Environ. Med. 76(10), 718–725 (2019).
Google Scholar
-
Virtanen, M. et al. The joint contribution of cardiovascular disease and socioeconomic status to disability retirement: A register linkage study. Int. J. Cardiol. 1(230), 222–227 (2017).
Google Scholar
-
Kouwenhoven-Pasmooij, T. A., Burdorf, A., Roos-Hesselink, J. W., Hunink, M. G. M. & Robroek, S. J. W. Cardiovascular disease, diabetes and early exit from paid employment in Europe; The impact of work-related factors. Int. J. Cardiol. 15(215), 332–337 (2016).
Google Scholar
-
Timmis, A. et al. European society of cardiology: Cardiovascular disease statistics 2019. Eur. Heart J. 41(1), 12–85 (2020).
Google Scholar
-
Salonen, P. Factors associated with premature departure from working life among ageing food industry employees. Occup. Med. (Chic Ill). 53(1), 65–68 (2003).
Google Scholar
-
Goetzel, R. Z. et al. The relationship between modifiable health risk factors and medical expenditures, absenteeism, short-term disability, and presenteeism among employees at novartis. J. Occup. Environ. Med. 51(4), 487–499 (2009).
Google Scholar
-
Sewdas R, De Wind A, Stenholm S, Coenen P, Louwerse I, Boot C, et al. Association between retirement and mortality: Working longer, living longer? A systematic review and meta-analysis. Vol. 74, Journal of Epidemiology and Community Health. BMJ Publishing Group; 2020. p. 473–80.
-
Strandberg, T. E. et al. Cohort profile: The Helsinki businessmen study (HBS). Int. J. Epidemiol. 45(4), 1074–1074h (2016).
Google Scholar
-
Keys, A. et al. Probability of middle-aged men developing coronary heart disease in five years. Circulation. 45(4), 815–828 (1972).
Google Scholar
-
Piepoli, M. F. et al. European guidelines on cardiovascular disease prevention in clinical practice the sixth joint task force of the European society of cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representative of 10 societies and by invited experts. Developed with the special contribution of the European association for cardiovascular prevention & rehabilitation). Eur. J. Prev. Cardiol. 23(11), 1–96 (2016).
Google Scholar
-
Hyttinen, L. et al. Effect of cholesterol on mortality and quality of life up to a 46-year follow-up. Am J. Cardiol. 108(5), 677–681 (2011).
Google Scholar
-
Strandberg, T. E. et al. One-hour glucose, mortality, and risk of diabetes: A 44-year prospective study in men. Archiv. Intern. Med. 171(10), 941–954 (2011).
Google Scholar
-
Aktas, M. K., Ozduran, V., Pothier, C. E., Lang, R. & Lauer, M. S. Global risk scores and exercise testing for predicting all-cause mortality in a preventive medicine program. JAMA 292(12), 1462–1468 (2004).
Google Scholar
-
Haapanen, M. J. et al. Retirement age and type as predictors of frailty: A retrospective cohort study of older businessmen. BMJ Open 10(12), 37722 (2020).
Google Scholar
-
von Bonsdorff, M. B. et al. Work-loss years among people diagnosed with diabetes: A reappraisal from a life course perspective. Acta Diabetol. 55(5), 485–491 (2018).
Google Scholar
-
R Core Team. R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2020.
-
Therneau, T. M. Survival Analysis [R package survival version 3.5–5]. 2023 Mar 12;
-
CRAN – Package extrafont [Internet]. [cited 2023 Apr 3]. Available from: https://cran.r-project.org/web/packages/extrafont/index.html
-
Halford, C. et al. Effects of self-rated health on sick leave, disability pension, hospital admissions and mortality. A population-based longitudinal study of nearly 15,000 observations among Swedish women and men. BMC Public Health 12, 1103 (2012).
Google Scholar
-
Pietiläinen, O., Laaksonen, M., Rahkonen, O. & Lahelma, E. Self-rated health as a predictor of disability retirement–the contribution of ill-health and working conditions. PloS One 6(9), e25004 (2011).
Google Scholar
-
De Breij, S. et al. Educational differences in the influence of health on early work exit among older workers. Occup. Environ. Med. 77(8), 568–575 (2020).
Google Scholar
-
Kiiskinen, U., Vartiainen, E., Puska, P. & Aromaa, A. Long-term cost and life-expectancy consequences of hypertension. J. Hypertens. 16(8), 1103–1112 (1998).
Google Scholar
-
Hird, T. R. et al. Productivity burden of hypertension in Australia: A life table modeling study. Hypertension 73(4), 777–784 (2019).
Google Scholar
-
Ademi, Z. et al. The economic impact of familial hypercholesterolemia on productivity. J. Clin. Lipidol. 14(6), 799–806 (2020).
Google Scholar
-
Schofield, D. et al. The costs of diabetes among Australians aged 45–64-years from 2015 to 2030: Projections of lost productive life years (PLYs), lost personal income, lost taxation revenue, extra welfare payments and lost gross domestic product from Health&WealthMOD2030. BMJ Open 7(1), 13158 (2017).
Google Scholar
-
Dall, T. M. et al. The economic burden of elevated blood glucose levels in 2012: Diagnosed and undiagnosed diabetes, gestational diabetes mellitus, and prediabetes. Diabetes Care 37(12), 3172–3179 (2014).
Google Scholar
-
Strandberg, T. E. et al. Low cholesterol, mortality, and quality of life in old age during a 39-year follow-up. J. Am. Coll. Cardiol. 44(5), 1002–1008 (2004).
Google Scholar
-
Strandberg, A. Y. et al. Low midlife blood pressure, survival, comorbidity, and health-related quality of life in old age: The Helsinki Businessmen study. J. Hypertens. 32(9), 1797–1804 (2014).
Google Scholar
Funding
MJH is supported by Finska Läkaresällskapet r.f. and Liv och Hälsa r.f. The Academy of Finland supported MBvB with grant 257239 and 349336. The Academy of Finland supported TT with grant 286536. The Academy of Finland supported MEvB with grants 294530, 307114 and 303920. TES is supported by the Helsinki University Hospital, Department of Medicine.
Author information
Authors and Affiliations
Contributions
M.J.H. designed the present study, drafted the article, analyzed the data, and drafted the manuscript. T.T. designed the present study, analyzed the data, interpreted the data, and revised the article critically for important intellectual content. M.B.B. designed the present study, interpreted the data, and revised the article critically for important intellectual content. M.E.B. and A.Y.S. interpreted the data and revised the article critically for important intellectual content. T.E.S. acquired funding for the cohort, data collection, interpreted the data and revised the article critically for important intellectual content.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary Tables.
Supplementary Figure 1.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
Reprints and Permissions
About this article
Cite this article
Haapanen, M.J., Törmäkangas, T., von Bonsdorff, M.E. et al. Midlife cardiovascular health factors as predictors of retirement age, work-loss years, and years spent in retirement among older businessmen.
Sci Rep 13, 16526 (2023). https://doi.org/10.1038/s41598-023-43666-x
-
Received: 13 June 2023
-
Accepted: 27 September 2023
-
Published: 02 October 2023
-
DOI: https://doi.org/10.1038/s41598-023-43666-x
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.