Cardiovascular
New Drugs May Help Rebuild Momentum against Cardiovascular Disease
By Tiffany Yesavage, PhD
We can gauge progress against cardiovascular disease by poring over statistics. But which statistics? We hear that cardiovascular disease is still the leading cause of death, but by less of a margin than previously. The narrowing of cardiovascular disease’s relative lead sounds encouraging, especially since the trend has held for about 50 years. Still, we may wonder if cardiovascular disease is receding or if other causes of death, such as cancer and respiratory disease, are advancing.
Perhaps a more telling statistic is premature heart disease mortality. It was front and center in a recent study led by researchers based at the U.S. Centers for Disease Control and Prevention (Trends Cardiovasc Med. 2020; 30(6): 364–374). The researchers indicated that the premature heart disease mortality rate among adults aged 25–64 had decreased by 70% since 1968, but that it had remained “stagnant” since 2011. Worse, by the end of the study period, heart disease still accounted for almost one in five of all deaths in the selected age group.
Essentially, the researchers told a story of momentum gained, then lost. Nonetheless, the researchers sounded an optimistic note. They wrote, “Public health and clinical systems can implement proven effective strategies and innovative promising practices across the spectrum of heart disease prevention.”
In their article, the researchers emphasized the proven strategies. For example, they cited the identification and management of key cardiovascular disease risk factors, as well as advances in medical treatment. The latter include the widespread adoption of cholesterol-lowering statins, arterial stents, and thrombolytic agents to break up blood clots.
Less was said about new innovations. But these merit attention, too. So, let’s look at just a few of them. Continue reading this GEN article to learn what four pioneering companies are doing to help the medical establishment rebuild momentum against heart disease and cardiovascular disease in general.
Targeted delivery of gene therapy to heart cells
Sharif Tabebordbar, PhD, chief scientific officer of Kate Therapeutics, recalls watching his father battle a rare genetic muscle disease called facioscapulohumeral muscular dystrophy. He remembers that the struggle, which lasted years, prompted him to think, “How can we make life better for my dad?” The experience inspired him to study genetics.
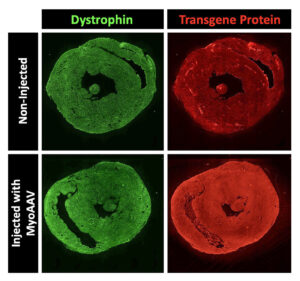
Nowadays, Tabebordbar hopes to improve the delivery of gene therapy. He focuses on altering the protein shell—called a capsid—of adeno-associated viruses (AAVs). These nonpathogenic viruses serve as vectors for transporting gene therapy to various tissues in the body. Each viral particle contains the cargo—the gene you want to deliver.
However, the main issue with injecting naturally occurring AAV capsids into the bloodstream is that most of these viruses are shuttled to the liver tissue. “Only a small percentage will actually get to the muscles or heart tissue,” Tabebordbar observes. “Therefore, you need very high levels of virus to have an effect on those tissues.” Unfortunately, elevated viral doses have been associated with severe liver toxicity and even death in recent clinical trials.
Over the past few years, there has been a significant focus on developing new modalities that will deliver gene therapy to specific tissues. In the case of naturally occurring AAVs, the proteins that form their surface structure determine which tissues of the body they can enter. These proteins can be modified and engineered using a technology called directed evolution.
Tabebordbar says that Kate Therapeutics is leveraging directed evolution approaches to engineer AAV capsids targeting muscles and heart tissues. However, he warns that an additional challenge with cardiovascular gene therapy involves ensuring homogenous delivery into heart muscle cells. “You don’t want drugs in just one part of the heart and not the other parts,” he stresses. “The pumping and electrical functions of the heart depend on the consistent, synchronized activity of all cardiomyocytes.”
Kate Therapeutics hasn’t disclosed the exact modalities and indications it is pursuing. Nonetheless, the company indicates that it aims to treat several rare genetic diseases. According to Tabebordbar, Kate Therapeutics will realize its goals by leveraging its ability to deliver gene therapy at a much lower dose. “This is going to result in much better safety profiles,” he declares. “That way, we can achieve the same gene expression levels in heart and muscle cells without having to worry about liver toxicity.”
Antithrombotic effects without bleeding
Platelets are tiny cell fragments in the blood responsible for natural coagulation, the process that stops cuts from bleeding. In contrast, cerebral thrombosis is the pathological mechanism of coagulation—in the form of a blood clot—that manifests during ischemic strokes.
“Unfortunately, most drugs that inhibit platelet aggregation also tend to cause bleeding,” says Gilles Avenard, MD, the CEO of Acticor Biotech. He explains that Acticor has developed a novel drug called glenzocimab—a humanized monoclonal antibody fragment—that disaggregates platelets without associated bleeding.

Glenzocimab is designed to inhibit a receptor on the surface of platelets called glycoprotein VI (GPVI), which binds to polymerized collagen and fibrin and promotes coagulation. In mice, deficiencies in GPVI have been found to be protective against thrombosis.
“The beauty of our drug,” Avenard maintains, “is that it targets thrombosis while also reducing inflammation downstream of the blood clot. This inflammation is thought to be due to small aggregates that dissolve the blood-brain barrier downstream of the clot, leading to intracerebral hemorrhages.”
Avenard explains that there are two major treatments for acute ischemic stroke: the administration of a drug called tissue plasminogen activator (tPA) and a procedure called a mechanical thrombectomy, which involves using a catheter to pull clots out of the cerebral artery. However, these treatments have both been associated with an increased risk of intracerebral hemorrhage.
Avenard reports that during Phase I/II trials to assess the safety of glenzocimab on top of tPA, investigators were pleasantly surprised to see that the treated group experienced fewer intracerebral hemorrhages than the placebo group. A Phase II/III study also involved administering the drug on top of tPA and thrombectomy. Whereas none of the patients in the treatment group died of an intracerebral hemorrhage, four patients in the placebo group (tPA and thrombectomy alone) died from bleeding.
According to Avenard, investigators expect to see similarly favorable results in a larger upcoming trial involving 1,000 patients worldwide. In addition to ischemic strokes, Acticor is planning future trials to target heart attacks and pulmonary embolisms.
Targeting microRNA to treat heart failure
Although noncoding RNA is not translated into proteins, it plays a crucial role in regulating many cellular processes. Notably, the dysregulation of noncoding RNA has been associated with human diseases, including cancer and heart failure.
Noncoding RNA figures in the work of Cardior Pharmaceuticals. “We have identified a crucial noncoding RNA called microRNA-132 that is responsible for the detrimental remodeling of heart cells,” says Thomas Thum, MD, PhD, the company’s co-founder and chief scientific officer. “We have documented how increases in microRNA-132 drive cardiac disease processes leading to heart failure.”

Thum explains that Cardior has developed CDR132L, a microRNA-132 inhibitor that specifically recognizes and “blocks” aberrant microRNA-132 levels. “CDR132L reverses cellular pathology, restoring normal heart muscle cells and overall heart function,” he explains. Thus far, the disease-modifying effects of the drug have been demonstrated in several animal models and a Phase Ib trial in chronic heart failure patients.
Cardior is also launching two Phase II trials to further assess the safety and efficacy of CDR132L. One trial is already enrolling participants with reduced left ventricular ejection fraction following a heart attack—an indication that the heart muscles are not pumping as well as normal. The other Phase II trial, set to begin enrollment in 2024, will investigate chronic heart failure in U.S. patients.
Thum stresses the importance of monitoring ongoing and future trials for the possibility of drug toxicity. Nevertheless, he says that the company’s Phase Ib study, conducted on top of the standard of care, showed no safety concerns or unexpected adverse effects, even at higher doses. He asserts, “This outcome, in addition to our extensive preclinical research into safety, supports our belief that CDR132L is a safe treatment option.”
Limiting tissue damage during heart attacks
During a heart attack, a blood clot forms within a coronary artery, reducing blood supply to specific areas of the heart. The affected region then becomes vulnerable to developing an infarct—a localized region of dead tissue caused by insufficient blood circulation.
Heart attacks prompt a series of reactions in the body referred to as adrenergic stress, leading to increases in heart rates and the force of the heartbeat—known as contractility. And while stenting procedures often help to widen constricted coronary arteries associated with heart attacks, they also increase adrenergic stress.
Kjetil Hestdal, MD, PhD, the CEO of Serca Pharmaceuticals, explains that the company is developing a drug called 13-M that disables the effects of adrenergic overstimulation in the heart: “By reducing contractility, the heart’s muscle cells in the vulnerable area surrounding the infarct will experience a lower resting workload. This will reduce the overall energy demand and prevent the death of these cells.” He also emphasizes that reductions in infarct size are correlated with a lower risk of developing heart failure following a heart attack.
Hestdal says that 13-M—currently in the preclinical development stage—is a new chemical entity that came from the laboratory of Kjetil Taskén, MD, PhD, a professor of medicine at the University of Oslo. Taskén’s team developed a sensitive, high-throughput assay to screen over 80,000 compounds for the ability to block a critical pathway in the adrenergic stimulation of heart muscle called SERCA2. “The SERCA2 pump,” Hestdal points out, “regulates calcium flow in the heart muscle cells and plays a key role in myocardial contractility.”
Hestdal says that 13-M will be administered intravenously before and after stenting procedures. He adds, “To our knowledge, no other drug in development has the same cardioprotective target.” He explains that the key behind 13-M is that it works by modulating contractility while maintaining heart rate: “This combination reduces the chances of inducing unintended disturbances in heart rate that could potentially lead to serious heart arrhythmias or heart fibrillation.” In contrast, drugs such as beta blockers lower both heart rate and contractility.
Looking toward the future
Avenard stresses that although emerging therapies like gene editing are very exciting, emergency treatments will always be necessary for heart attacks and strokes. Meanwhile, Thum argues that many cardiovascular drugs on the market today tend to alleviate symptoms without addressing underlying root causes. He notes, however, that “with new treatment modalities like microRNA therapy, we can target downstream pathways that regulate critical processes in the pathology.”
Finally, Tabebordbar says that Kate Therapeutics is hoping to target several genetic diseases over the next few years, including his father’s condition. Although he acknowledges that “there are still a lot of safety concerns to overcome,” he emphasizes that “gene therapy and gene editing ultimately have the potential to treat broader cardiovascular conditions like high cholesterol and heart failure.”