Congenital disorders
Topical Ketamine: A Promising New Avenue for PTSD Treatment
A new clinical trial is underway to test a ketamine topical cream designed to alleviate the symptoms of post-traumatic stress disorder (PTSD), without causing the psychedelic adverse effects (AEs) often associated with other forms of ketamine administration. Ketamine topical (NeuroDirect; Psycheceutical Bioscience) could provide a safer, non-systemic way to address debilitating mental health issues.
In a clinical trial testing the topical ketamine therapy, investigators successfully dosed their first cohort of subjects in a phase I clinical trial evaluating the safety, tolerability, and pharmacokinetics of a single dose of ketamine topical in 24 healthy volunteers. Key results from the study confirmed the topical administration to be safe and well-tolerated.1 The clinical trial program is the first ever to test the topical application of ketamine for use in treating a mental health disorder, and phase II clinical trials are planned for the beginning of 2024.
“We are excited about the overall success of our first cohort from our phase I clinical trial,” said Chad Harman, CEO of Psycheceutical, in a press release. “The favorable safety profile for our [topical ketamine] represents an exciting milestone in our goal of bringing to market a novel method for treating mental health and neurodegenerative diseases. We look forward to expanding our drug pipeline with other psychedelic compounds using this novel delivery method in the near future.”
Benefits of Topical Application
The topical application of ketamine offers several potential benefits over systemic delivery methods. First and foremost, a topical ketamine approach reduces the risk of AEs commonly associated with other forms of ketamine delivery, such as nausea or hallucinations.2
Moreover, the convenience of topical application cannot be overstated. Unlike intravenous (IV) administration, which requires medical supervision and can be costly, topical ketamine can be self-administered at home.1 This makes it a more accessible and practical treatment option for many patients who struggle with mental health issues.
Traditional ketamine delivery methods such as capsules, IV infusions, and sublingual troches often come with significant risks. These methods involve systemic administration, resulting in active medication circulating the bloodstream to affect the function of vital peripheral organs. Even at low doses, this can lead to short-term AEs like increased heart rate and blood pressure, disorientation, dizziness, nausea, and vomiting, as well as psychedelic effects such as dissociation and hallucinations.3 Oral routes of administration can lead to an unpredictable onset of effects, as the absorption rate in the digestive system can vary greatly by patient.4 In addition, IV administration necessitates medical oversight and is typically performed in a clinical environment, which can be both inconvenient and expensive.5
Long-term use of ketamine through systemic administration may also result in liver and kidney damage, and lead to physical dependence and associated withdrawal symptoms such as depression, excessive sleepiness, and drug cravings.6 Long-term systemic abuse may also lead to neurological risks including memory impairments and declines in executive functioning.7
Non-Systemic Delivery of Ketamine
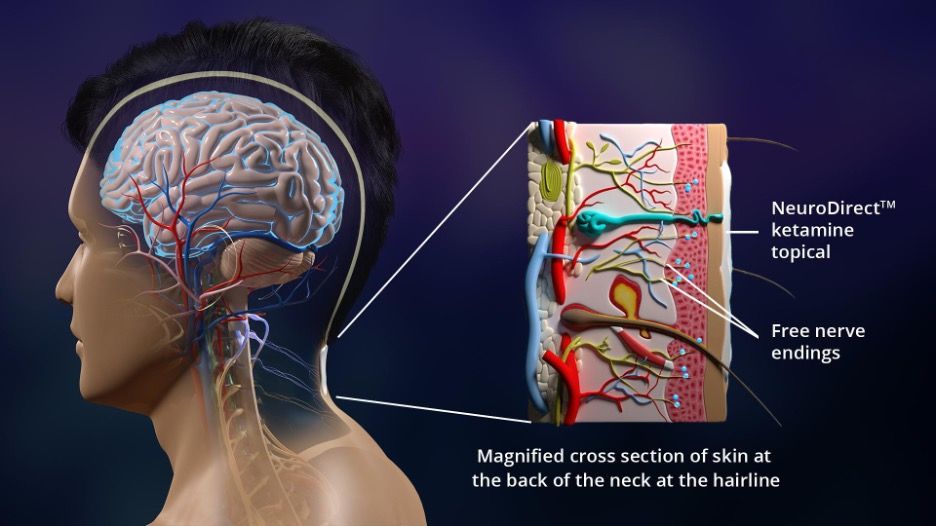
How Ketamine Topical Works: Applied to the back of the neck at the hairline, ketamine topical activates receptors on free nerve endings, inducing neurochemical reactions which transmit nerve action potentials to the brain. Image Credit: © Psycheceutical Bioscience
The topical delivery technology is designed to take advantage of the non-systemic benefits of ketamine to rapidly relieve symptoms of neuropsychiatric conditions. The topical cream is applied to the back of the neck at the hairline to deliver neuroaffective compounds directly to the nervous system. Topical ketamine activates receptors on free nerve endings under the skin’s surface at this critical location adjacent to the brainstem. Here, neurochemical reactions communicate with the brain to promote therapeutic benefits without the restrictions of the blood-brain barrier. This drug delivery method produces therapeutic benefits within minutes, while reducing or eliminating the AEs commonly associated with other forms of ketamine delivery such as hallucinations, nausea, and dizziness.
Because topical ketamine is a non-invasive application with a low concentration of the active ingredient, the potential for abuse is limited. Further, in designing topical ketamine to be administered at home instead of in a clinical setting, the company hopes to greatly lower the cost-of-care for both insurance companies and patients, thereby increasing access to these life-saving treatments for anyone suffering from mental health disorders.
Promising Observational Clinical Data
The ketamine topical cream has already shown positive results in an observational clinical setting. Ron Aung-Din, MD, inventor of the NeuroDirect delivery system and clinical advisor to Psycheceutical, recently published a peer-reviewed study in Drug Development & Delivery which describes the potential of the ketamine topical cream as an improved treatment for PTSD. In 100 patients who had failed numerous other treatments for intractable depression, anxiety, and other symptoms commonly associated with PTSD, more than 80% experienced symptom relief after using the ketamine cream. No psychogenic or other deleterious systemic AEs were observed.8
“Discernible improvement[s] in anxiety, depression, paranoia, and unrealistic fear, focusing issues, cloudy thinking, neuropathic pain, and other such symptoms were noted within 8 to 10 minutes of topical drug application,” wrote Aung-Din in Drug Development & Delivery. “No psychogenic effects, such as hallucinations or dissociative phenomena, were experienced by any patient. To the contrary, patients indicated their thought processes were clearer, more focused, and that they were more keenly aware of surroundings.”
Hope for the Future
Ketamine has recently emerged as a breakthrough treatment for several mental health disorders. Clinicians and researchers have discovered its potential to provide rapid relief for conditions such as depression, anxiety, and PTSD, particularly in patients who haven’t responded to conventional therapies.9 As both an off-label treatment and in the form of an FDA-approved nasal spray, ketamine is beginning to change the clinical landscape for mental health treatment, offering hope to those grappling with persistent psychological challenges.
As both an off-label treatment and in the form of an FDA-approved nasal spray, ketamine is beginning to change the clinical landscape for mental health treatment, offering hope to those grappling with persistent psychological challenges. Image Credit: © Julia – stock.adobe.com

However, concerns about ketamine remain, not only due to its history as a party drug and potential for abuse, but also for inducing “trippy” psychedelic effects such as hallucinations, dissociation, dizziness, and nausea. These issues have created barriers to its acceptance in the medical community and among patients.
The development of topical ketamine represents an exciting advancement in the realm of mental health treatment. Its potential to provide relief from mental health symptoms with fewer AEs and greater accessibility could make it a valuable tool for the medical community.
About the Author
Aric Logsdon, PhD, received his doctorate in Pharmaceutical Sciences from West Virginia University. Logsdon is currently a postdoctoral fellow in the Department of Psychiatry at the University of Washington School of Medicine
References
- Fultinavičiūtė U. Psycheceutical starts a Phase I trial with topical ketamine. Clinical Trials Arena. https://www.clinicaltrialsarena.com/news/psycheceutical-neurodirect-ketamine/. Published July 14, 2023.
- Houser K. New clinical trial is testing a ketamine skin cream for PTSD. Freethink. https://www.freethink.com/health/topical-ketamine-for-ptsd. Published July 26, 2023.
- Drug fact sheet: Ketamine. Department of Justice, Drug Enforcement Administration. April 2020.
- Research C for DEA. FDA alerts health care professionals of potential risks associated with compounded ketamine nasal spray. US Food And Drug Administration. February 2022. https://www.fda.gov/drugs/human-drug-compounding/fda-alerts-health-care-professionals-potential-risks-associated-compounded-ketamine-nasal-spray.
- Ketamine use for Treatment resistant Depression and Pain – Compounding Pharmacy Los Angeles – Enovex. Compounding Pharmacy Los Angeles – Enovex. https://enovexrx.com/health-articles/ketamine-use-for-treatment-resistant-depression-and-pain/.
- Treatment Improvement Protocol—TIP 45: Detoxification and Substance Abuse Treatment. Substance Abuse and Mental Health Services Administration. 2015.
- Strous, Weeland, Van Der Draai, et al. Brain changes associated with long-term ketamine abuse: A systematic review. Frontiers in Neuroanatomy. 2022;16:1-17.
- Marino D. DELIVERY TECHNOLOGY – NeuroDirect™ Ketamine: Novel, Non-Systemic Topical Therapy for PTSD & Associated Intractable Depression. Drug Development and Delivery. March 2023. https://drug-dev.com/delivery-technology-neurodirect-ketamine-novel-non-systemic-topical-therapy-for-ptsd-associated-intractable-depression/. Culp CJ, Kim HK, Abdi S. Ketamine use for cancer and chronic pain management. Frontiers in Pharmacology. 2021;11. doi:10.3389/fphar.2020.599721