Infection
#VisualAbstract: Prophylactic antibiotics prevent urinary tract infections in infants with vesicoureteral reflux
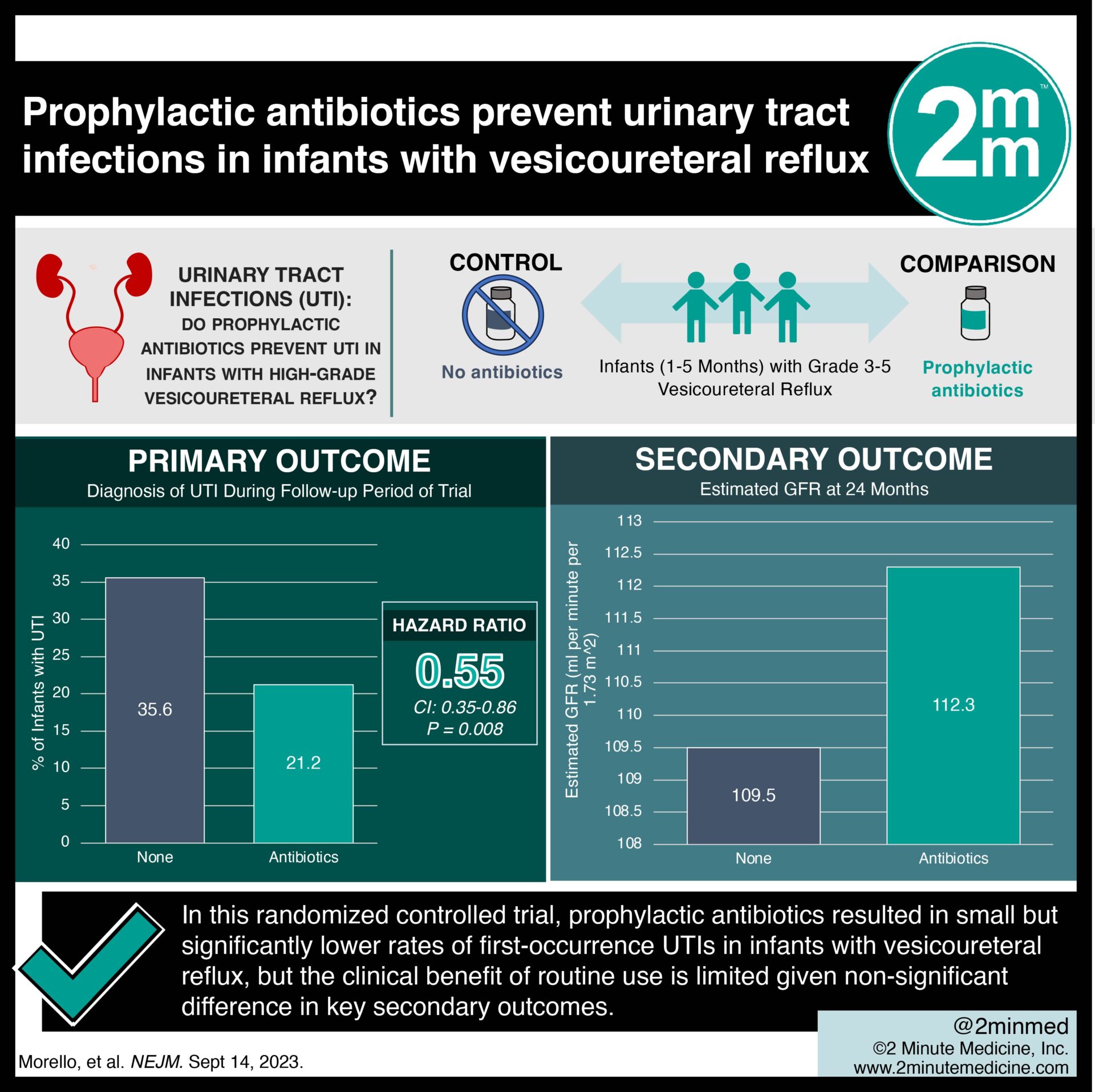
 1. In this randomized controlled trial, prophylactic antibiotics resulted in significantly lower rates of first-occurrence urinary tract infections (UTIs) in infants with vesicoureteral reflux.
1. In this randomized controlled trial, prophylactic antibiotics resulted in significantly lower rates of first-occurrence urinary tract infections (UTIs) in infants with vesicoureteral reflux.
2. The groups did not differ in kidney scarring, kidney filtration function, or hospitalization rates due to UTI.
Evidence Rating Level: 1 (Excellent)
Study Rundown: Infants with vesicoureteral reflux are at an elevated risk of developing UTIs. This increases their cumulative risk of adverse events such as kidney scarring and reduced filtration function. To date, there has been limited research into the benefits of prophylactically providing antibiotics to infants with high-grade vesicoureteral reflux who have not previously had a UTI. This was a randomized open-label trial involving multiple centers across Europe investigating the effectiveness of prophylactic antibiotics for infants with high-grade vesicoureteral reflux without previous UTI. Results of the study found that providing prophylactic antibiotics did reduce the rate of first UTI in infants compared to those who did not receive prophylactic antibiotics. However, there were no differences in secondary outcomes such as kidney scaring, filtration function, or hospitalization rates. Further, a large portion of the no antibiotics group did not develop UTIs, and the prophylactic antibiotics group demonstrated greater antibiotic resistance microbes. This study is limited by confounding effects introduced by the lack of a single standardized antibiotic. Overall, it provided evidence that antibiotic prophylaxis may reduce first UTIs in infants with vesicoureteral reflux. However, there may be no change in long-term outcomes.
Click to read the study in NEJM
In-Depth [randomized controlled trial]: This was a multisite, randomized trial investigating the effectiveness of 24 months of prophylactic antibiotics for infants with vesicoureteral reflux in preventing the incidence of UTIs. The primary outcome of interest was a diagnosis of UTI during the follow-up period of the trial. Additional outcomes of interest included the occurrence of kidney scarring, kidney function by proxy of estimated glomerular filtration rate at the end of the trial period, and incidence of hospitalization due to UTI. Infants between the ages of one to five months with voiding cystourethrography- or ultrasonography-confirmed grade three to five vesicoureteral reflux were included in the study. Infants younger than 35 weeks gestational age, those with a previously diagnosed UTI, known ureteropelvic junction or ureterovesical junction obstruction, neurogenic bladder, or posterior urethral valves were excluded from the study. After applying the inclusion and exclusion criteria, 292 infants were randomly assigned in a 1:1 ratio to receive 24 months of prophylactic antibiotics or no antibiotics. Primary results of the analysis found that prophylactic antibiotics significantly reduced the rate of the first occurrence of UTI compared to no antibiotics (hazard ratio 0.55; 95% confidence interval, 0.35-0.86; p=0.008). The prophylactic antibiotic group demonstrated a greater incidence of antibiotic resistance than the untreated group. The overall number needed to treat to prevent a UTI episode was seven. There was no difference in secondary outcomes or adverse events between the groups. Although this trial demonstrated a small and significant benefit of continuous prophylactic antibiotics, the clinical benefit of routine use in this population is limited given the non-significant difference in key secondary outcomes.
©2023 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.