Blood
Nanotechnology helps chemo pass the blood-brain barrier
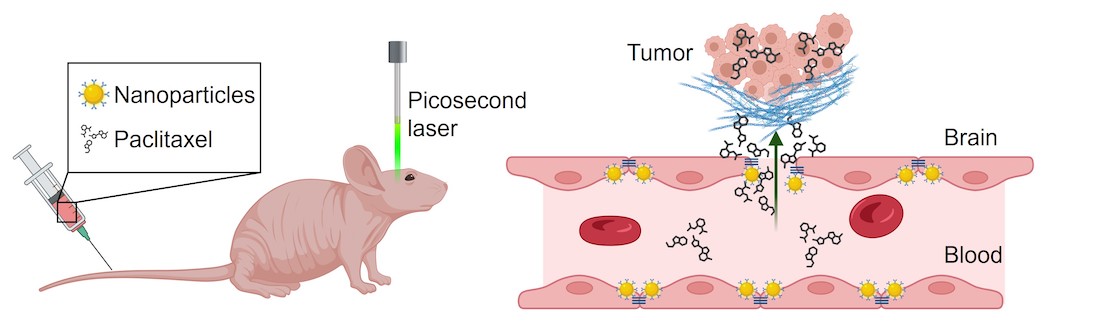
Infiltrative glioma cells are protected against commonly used anti-cancer drugs like paclitaxel, which cannot cross the blood-brain barrier (BBB). Using mouse models, UT Southwestern and UT Dallas researchers coated gold nanoparticles (NP) with an antibody targeting them to the brain endothelium. Picosecond laser stimulation of the NP through an intact skull caused a vibration excitation of the NP, opening the BBB without causing injury to the delicate brain tissue and allowing the drug to enter the brain and either kill or block the growth of tumor cells. (Image created with BioRender.com)
DALLAS – Oct. 16, 2023 – Combining a common chemotherapy drug with an experimental nanotechnology allowed the drug to cross the blood-brain barrier and increased the survival rate in a mouse model of glioblastoma up to 50%, a team led by researchers from UT Southwestern Medical Center and UT Dallas found. Their research, published in Nature Communications, could lead to possible new treatments for glioblastoma, the most common and aggressive primary brain tumor.
“The biggest barrier to effectively treating glioblastoma has always been getting chemotherapies into the brain. This new approach could be a game changer for this disease,” said corresponding author Robert Bachoo, M.D., Ph.D., Associate Professor of Neurology and Internal Medicine and a member of the Harold C. Simmons Comprehensive Cancer Center at UT Southwestern. Zhenpeng Qin, Ph.D., Adjunct Associate Professor of Biomedical Engineering at UTSW and an Associate Professor at UT Dallas, also was a corresponding author.

Robert Bachoo, M.D., Ph.D., Associate Professor of Neurology and Internal Medicine and a member of the Harold C. Simmons Comprehensive Cancer Center at UT Southwestern, is a corresponding author on the study. Dr. Bachoo holds the Miller Family Professorship in Neuro-Oncology.
Despite decades of research, the prognosis for glioblastoma patients remains dismal; their median survival rate is about 15 months after diagnosis. Although surgery can remove the primary tumor, glioblastoma almost universally returns due to cancerous cells that spread beyond the tumor’s visible margins.
The biggest obstacle to killing these cells is the blood-brain barrier (BBB), proteins that form tight junctions between cells that line the brain’s blood vessels and prevent potentially toxic molecules from entering the brain. Although many chemotherapy drugs are effective against glioblastoma cells in petri dishes, Dr. Bachoo explained, the BBB prevents these drugs from reaching glioblastoma cells in patients and animal models.
Researchers have explored several strategies to open the BBB, but each has significant risks, including excessive toxicity and heat damage.
In the new study, Dr. Bachoo and his colleagues tested a new approach: gold nanoparticles coated with antibodies against a key protein in the BBB complex, a strategy they named optoBBTB. When these nanoparticles are injected intravenously in animal models, they migrate and attach to the tight junction proteins. A precise wavelength of laser light can cause these nanoparticles to vibrate, opening the BBB without generating heat.
The researchers worked with two types of genetically engineered mice that have mutations found in human glioblastoma patients and recapitulate key features of glioblastoma. When the researchers delivered optoBBTB to mice of either type, dye injected shortly afterward infiltrated the animals’ tumors, suggesting this strategy successfully breached the BBB.
Subsequent tests using the chemotherapy drug paclitaxel – also known under the brand name Taxol and used as a first-line treatment in a variety of cancers – showed it readily infiltrated the animals’ tumors after optoBBTB. After three cycles of paclitaxel treatment with optoBBTB delivered three days apart, the mouse models’ tumors shrank up to sevenfold, and the mice lived up to 50% longer compared with animals that received intravenous Taxol or a placebo.
Although these survival gains are significant, Dr. Bachoo said, the real promise behind optoBBTB is its ability to allow a variety of other promising chemotherapy drugs to be used on the brain, 98% of which cannot currently cross the BBB.
Other UTSW researchers who contributed to this study include Elizabeth Maher, M.D., Ph.D., Professor of Internal Medicine and Neurology and member of the Simmons Cancer Center, and Vamsidhara Vemireddy, M.D., Senior Research Scientist. First author Qi Cai, Ph.D., was a postdoctoral fellow in Dr. Qin’s UT Dallas laboratory.
Dr. Bachoo holds the Miller Family Professorship in Neuro-Oncology. Dr. Maher holds the Theodore H. Strauss Professorship in Neuro-Oncology.
This research was funded by the Cancer Prevention and Research Institute of Texas (CPRIT) (RP190278 and RP210236), the Department of Defense (W81XWH-21-1-0219), the American Heart Association (19CSLOI34770004), the National Institutes of Health (RF1NS110499), the National Science Foundation (2123971), and funds from a Eugene McDermott Professorship.
About UT Southwestern Medical Center
UT Southwestern, one of the nation’s premier academic medical centers, integrates pioneering biomedical research with exceptional clinical care and education. The institution’s faculty members have received six Nobel Prizes and include 26 members of the National Academy of Sciences, 20 members of the National Academy of Medicine, and 14 Howard Hughes Medical Institute Investigators. The full-time faculty of more than 3,100 is responsible for groundbreaking medical advances and is committed to translating science-driven research quickly to new clinical treatments. UT Southwestern physicians provide care in more than 80 specialties to more than 120,000 hospitalized patients, more than 360,000 emergency room cases, and oversee nearly 5 million outpatient visits a year.