Blood
Solvita debuts new blood donor guidelines for LGBTQ men
New blood donation guidelines are in place at one of the Tri-State’s two blood donation agencies, and the second is nearly ready to roll out its updated procedures. The changes are in response to new guidance from the U.S. Food and Drug Administration on who is allowed to donate blood.
The FDA in May released new rules eliminating restrictions that specifically prohibited gay and bisexual men from donating. The following month Hoxworth and Solvita (formerly Community Blood Center) told WVXU they welcomed the changes and were beginning the process of updating their procedures to comply with the new guidance.
RELATED: Here’s how Hoxworth and Community Blood Center are responding to updated blood donation guidelines
The FDA issued a new series of “individual risk-based questions” that are the same for everyone who donates blood. Previously, only men who have sex with men (or women who have sex with men who have sex with men) had to wait three months before donating blood.
Now, Solvita says, “All are asked if they have had new and/or multiple sexual partners in the past three months. If either answer is yes, the behavior is considered higher risk, and they are then asked about the type of sexual contact.”
All blood donations are required by FDA regulations to be screened for various infectious diseases, including HIV, Hepatitis B and C, West Nile, and more.
The updated guidelines mean most gay and bisexual men in monogamous relationships will not have to refrain from sex in order to give blood.
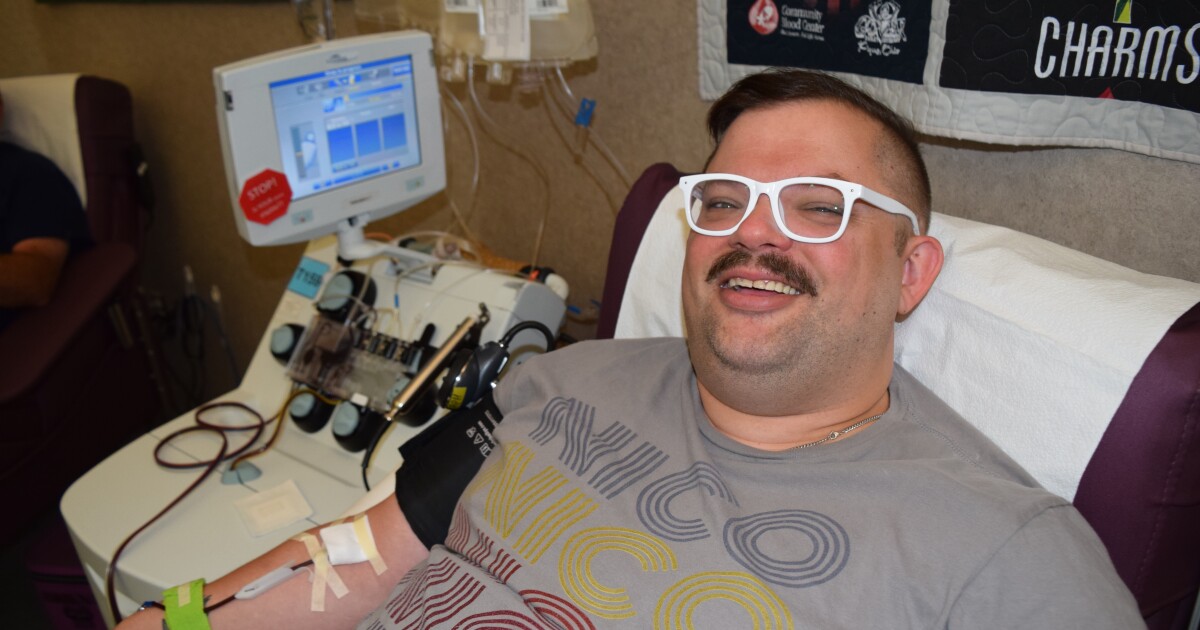
Dayton-based Solvita rolled out its new procedures Sept. 1. Nico Schrenk of West Carrollton was one of the first people in the door that day to give blood. It was his first blood donation since 2013.
“It’s in my blood to always give back to the community if you have the ability to help the community. That’s always been my motto,” Schrenk tells WVXU. “The reason why blood donation is such a passion for me is it’s literally taking 10 to 20 minutes out of your life, and helping so many people right here in our area.”
Schrenk, now a 37-year-old married gay man, came out in 2013. He says the process was difficult and sometimes violent as he fought critical and physical challenges from others and his own self doubt.
Coming out also meant he had to stop donating blood.
“I had to continually accept who I was, and in doing so, I had to accept the fact that I wouldn’t be able to donate blood anymore due to the FDA regulations. That was a very, very hard decision for me,” he says.
RELATED: More gay and bisexual men will now be able to donate blood under finalized FDA rules
Tears pricked at the corners of his eyes as he returned to the donation chair last month. He describes the moment as immensely powerful and filled with emotion, but adds there’s more work to be done.
“Yes, the FDA regulations lifting are beautiful and it’s a huge step, but we as the LGBT+ community are very passionate about our community we live in and for years and years and years and years hearing ‘We’re low on O positive,’ and me being O positive and not being able to donate… I’m right here. You just won’t take me because of regulations.
“It was very refreshing, very powerful, to be able to sit back and donate again.”
The decision was easy for him, but Schreck says he did think about how some people may be hesitant and/or angry over decades of being denied.
“I have to say that’s something that did cross my mind. ‘Why should I give back (and) support the people who denied us for so many years?’ But here’s the thing, it’s not about us; it’s not about them. It’s about the people in the hospital, our neighbors, our friends, who may be in the LGBT community as well. … I strongly encourage people to look past the politics and into how can you impact the community around you in a positive way.”
While the FDA regulations changed in May, it took time for blood banks like Solvita and Hoxworth to update their screening procedures and to train staff and volunteers on the new policies.
In a statement to WVXU, Hoxworth writes, “Hoxworth Blood Center is excited to welcome newly eligible blood donors to our mission of saving lives close to home. We are working across all departments in the blood center to implement the updated FDA changes, procedures, staff training, and computer system validations regulated by the FDA guidelines. This implementation process is in progress and is expected to go live for our Tri-State donors by early December.”
The blood center says more information can be found on its website.