Congenital disorders
A strong community fighting against weak muscles
Every Oct. 18, we celebrate a global community of patients, family, researchers and healthcare providers devoted to raising awareness of lamin A-related congenital muscular dystrophy. Their common goal is to educate the public about this disease and to advocate to lawmakers, philanthropists, and biotech and pharmaceutical companies for better diagnostic and treatment options.
What is L-CMD?
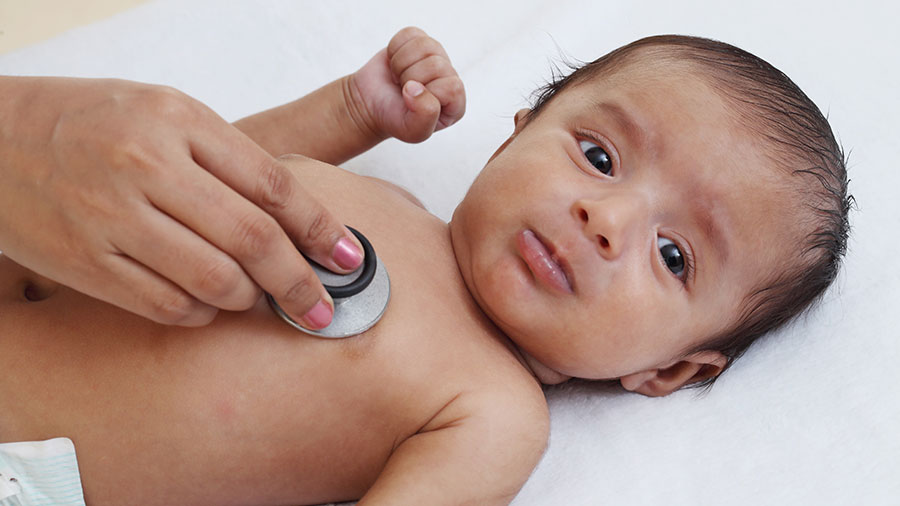
Congenital muscular dystrophy, or CMD, is the umbrella term for a diverse group of conditions that affect the striated muscles, causing muscle weakness at or near birth, often called “floppy baby syndrome.”
At the root of CMD is a genetic mutation affecting a critical protein for muscle structure and function; because many genes fit this description, there are many different forms of CMD with variable clinical manifestations and prognoses.
Mutations on the LMNA gene that codifies for lamin A and lamin C proteins are responsible for lamin A-related CMD (also known as L-CMD). Lamins are a group of proteins organized in a meshlike structure to reinforce the cell’s nuclear envelope and mechanically couple the nucleoskeleton and the cytoskeleton. The exact mechanism by which defective lamin A causes muscle weakness remains unknown.
L-CMD is inherited in an autosomal dominant manner, meaning that only one faulty copy of the gene is sufficient to trigger the disease. In all cases, these are new, spontaneous mutations that occur during the formation of reproductive cells or embryo development. This means that parental genetic testing cannot predict the occurrence of L-CMD. To date, around 100 cases have been reported in the literature.
L-CMB can manifest during the first year of a child’s development. Affected children have difficulty holding up their heads, rolling over and sitting without assistance.
How does L-CMD manifest?
Muscle hypotonia and wasting are early signs of L-CMD and can manifest during the first eight months of life. Affected babies cannot hold their heads up, roll over, hold large toys or sit up unassisted. In less severe forms, muscle weakness becomes apparent later, affecting mobility and feeding.
Some patients display abnormalities in spinal curvature and joints due to permanent muscle contractures. Patients with L-CMD can have heart problems and breathing difficulty due to the compromise of cardiac and respiratory muscles. Occasionally, the central nervous system is compromised, manifesting as developmental delays or cognitive impairment.
How is L-CMD diagnosed and treated?

Muscle biopsy from a patient with CMD stained with Hematoxylin and Eosin. Indication of muscle damage includes muscle fibers of variable size, central nuclei and inflammatory cells.
L-CMD should be suspected in otherwise healthy children with difficulties sitting and walking unassisted, joint deformities, and dropped-head syndrome, especially during the first decade of life.
Thus, assessing and documenting child developmental milestones is critical. Laboratory tests such as elevated creatine kinase levels can indicate muscle damage and can be accompanied by a muscle biopsy to evaluate tissue structure.
Confirmation of L-CMD requires genetic testing.
L-CMD has no cure, and patients die of cardiac or respiratory complications during their first or second decade. Treatment usually focuses on preserving motor skills for as long as possible and anticipating complications, which requires a knowledgeable and empathic multidisciplinary team.
Care for patients with CMD is also very expensive.
Progress on the horizon
Recent publications have shed light on the physiopathological mechanisms by which loss of lamin proteins can damage the skeletal muscles and the heart.
In an article published in the Journal of Biological Chemistry, researchers from Columbia University reported that in a mouse model of LMNA-related cardiomyopathy, the AKT-mTORC signaling pathway was hyperactivated in the heart, resulting in altered metabolism and energy deficit.
Because Lamin A/C is located in the inner nuclear membrane, it is believed to have a role during DNA metabolism. Scientists from St Louis University School of Medicine have reported that Lamin A/C is critical during DNA replication and that loss of it results in replication stress and genomic instability. Their findings were published in JBC too.
Researchers working in France and the U.K. reported that muscle cells derived from Lmna-CMD mice were highly dysfunctional and unable to grow or fuse in response to mechanical overload like normal muscle. They also confirmed similar abnormalities in biopsies from LMNA-CMD patients.

Nuclear envelope and related proteins (A) nuclear components including envelop with an outer nuclear membrane (ONM) and inner nuclear membrane (INM); (B) layers of the nuclear envelope, highlighting Lamin A/C and B; (C) nuclear envelope related proteins. Lamins-based mesh-like structure is depicted in blue/green. Notice its relationship with other envelope structures and with nuclear DNA.
Although advances have been made since the first reports about L-CMD about 15 years ago, researchers and families worldwide agree more needs to be done.
Researchers from Spain have been recently awarded funding from the organization Cure CMD to determine if genetic editing via CRISPR/Cas technology can alleviate cardiac abnormalities associated with L-CMD. By collaborating with scientists from UMass Chan School of Medicine, they are working towards making gene therapy a reality for L-CMD.
The power of patient-centered, nonprofit organizations
Nothing is more powerful than the courage and determination of those affected by rare diseases; CMD patients are no exception. They inspire a larger community of family, friends and caregivers who work relentlessly to make an impact.
Cure CMD is a nonprofit established in 2008 by a group of parents whose children had been diagnosed with CMD. Its mission is to “advance research toward treatments for CMD and improve the lives of those living with CMD through engagement and support of our community.”
In partnership with other nonprofit and scientific organizations, Cure CMD has awarded more than $4 million in research grants to scientists around the globe working on understanding and treating all forms of CMDs. Cure CMD also organizes regular scientific conferences, creates patient and family-oriented educational material, and keeps an up-to-date repository of scientific publications on CMD.
A similar journey, filled with heartbreaking and love in equal measure, led a Texas family to start the nonprofit L-CMD Research Foundation after the diagnosis of their youngest son, Austin. They are advocating for more and better basic research that will effectively and quickly translate into a cure for L-CMD, and they are on their way to raising $2 million to support this endeavor.
Other established organizations with decades of experience, such as the Muscular Dystrophy Association and the National Organization for Rare Disorders, also support research and outreach activities focused on L-CMD and CMDs.
To learn more about CMD or donate to research, please get in touch with the organizations listed above.