Cancer and neoplasms
Eccrine poroma with concurrent basal cell carcinoma
Department of Dermatology, Sinopharm Dongfeng General Hospital, Hubei University of Medicine, Shiyan, Hubei, People’s Republic of China
Correspondence: Jiao Wei; Xiao Xiong, Department of Dermatology, Sinopharm Dongfeng General Hospital, Hubei University of Medicine, Shiyan, Hubei, 442008, People’s Republic of China, Tel +86 15872706796 ; +86 13636169342, Email [email protected]; [email protected]
Abstract: Eccrine poroma (EP) is a benign skin appendicular tumor that differentiates into the terminal sweat duct and is often differentiated from basal cell carcinoma (BCC) and seborrheic keratosis. This report describes a 58-year-old woman who presented with left occipital plaque. Histopathological analysis showed that the tumor cells were located in the lower part of the epidermis. The tumor cells were cuboidal or circular basal-like cells of the same size. The surrounding cells were not arranged in a palisade shape. Scattered tumor clusters composed of basal-like cells were also seen in the dermis, staining basophilic, and the surrounding cells were arranged in a palisade pattern. Immunohistochemistry showed that BerEP4, epithelial membrane antigen EMA, carcinoembryonic antigen CEA, Bcl-2, CD10, CK7 were positive, AR, PAS were negative. According to the pathological examination and immunohistochemical results, a case of eccrine poroma with concurrent basal cell carcinoma was diagnosed.
Keywords: eccrine poroma, basal cell carcinoma, immunohistochemistry, carcinoembryonic antigen, CEA, epithelial membrane antigens, EMA, BerEP4
Introduction
Eccrine poroma (EP) is one of the pathologic types of poromas, a benign adnexal tumor with differentiation toward sweat pores (terminal ducts). Poromas can be divided into four types according to their pathological characteristics: dermal duct tumor, eccrine poroma, hidroacanthoma simplex, and poroid hidradenoma.1 The clinical manifestations of these types are similar and can only be distinguished by pathological diagnosis. But because their cell origins are similar, sometimes two or more subtypes can co-exist in a particular lesion.2,3 Basal cell carcinoma (BCC) is the most common malignancy of skin. BCC can be associated with a variety of skin diseases. Including non-neoplastic skin diseases such as vitiligo, Darier disease, granuloma faciale, dermatitis, varicose veins, scars, surgical sites, systemic infections, and ulcers.4 As well as neoplastic skin diseases such as nevus, seborrheic keratoses, melanoma and invasive melanoma.4 However, the cases of BCC complicated with EP are rarely reported worldwide.5 Here, we report a special case of eccrine poroma with concurrent basal cell carcinoma.
Case Presentation
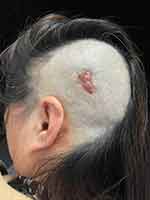
A 58-year-old Chinese woman presented with a progressive enlargement of red plaques in the left occipital area that had grown in size over the past two years with mild pruritus (Figures 1 and 2). Cutaneous examination revealed an irregular flesh-red plaque about 3.5×2.5 cm in size on the left occipital scalp, which slightly elevated to the surface of the skin, soft in texture and smooth in surface, with a few yellowish-brown crusts. Based on the clinical manifestations of this rash, we consider that it may be a basal cell carcinoma, poroma, keloid, verrucous epidermal nevus, or sebaceous nevus. We excised 5 mm safety margin around the plaque and performed skin biopsy.
|
Figure 1 Flesh-red plaque in the left occipital near the temporal. |
|
Figure 2 The red patch is about 3.5×2.5 cm in size, slightly raised on the surface of the skin, soft and smooth in texture, with a small amount of tan scab attached to it. Notes: The lesion was resected 5mm outside its margin, all divided into six pieces numbered 1–6 and sent for pathological biopsy. (All photographic materials were public with the patient’s consent). |
Histopathological analysis showed that the tumor cells were located in the lower epidermis and extended into the dermis in the shape of sheets and cords. Tumor cells are irregular basal-like cells of uniform size, smaller than normal keratinocytes. The nuclei are large and hyperchromatic. The tumor cells are closely arranged, but not palisading which located at the periphery. Ducts were also seen within the tumor nests. These findings established the diagnosis of EP. Basal-like cells were scattered around the cell mass of EP. The tumor nucleus is large, the cytoplasm is less, and the staining is basophilic. The cells surrounding the tumor cluster were arranged in a palisade pattern (Figure 3). Immunohistochemical study of the red plaque showed strong positive for BerEP4, positive for epithelial membrane antigens (EMA), carcinoembryonic antigen (CEA), B cell lymphoma/leukemia-2 (Bcl-2), Cluster of differentiation 10 (CD10), cytokeratin 7 (CK7), and negative for androgen receptor (AR). Periodic acid-Schiff stain (PAS) is negative (Figures 4 and 5).
|
Figure 3 Pathology features. Notes: (A and B) Tumors consist of uniformly sized basal-like cells that are smaller than normal keratinocytes and extend from the epidermis into the dermis in a ribbon. The tumor cells located at the periphery showed no palisading. The histologic findings were consistent with eccrine poroma (H&E, Bar: 500 μm, H&E, Bar: 50 μm). (C and D) Scattered basal-like cells were found in the dermis. The tumor nucleus is large, the cytoplasm is less, and the staining is basophilic. The cells around the tumor cluster are arranged in a palisade. The findings were consistent with basal cell carcinoma (H&E, Bar: 100 μm, H&E, Bar: 50 μm). |
|
Figure 4 Immunohistochemical expression in this plaque. Notes: BCC was positive for Bcl-2 (A), Ber-EP4 (B), CK7 (C), CD10 (D). Bars: 200 μm. |
|
Figure 5 Immunohistochemical stainings. Notes: (A) EMA was positive in the EP and negative in the BCC. (B) PAS was negative. (C) AR was negative. Bars: 200 μm. (D) CEA was positive in ductal cells of EP. Bar: 50 μm. |
According to the clinical and histopathological features, the final diagnosis was a EP with concurrent BCC. Pathological analysis of all tumor margins was negative. There was no recurrence during follow-up after treatment.
Discussion
EP was first described in 1956.6 It usually occurs in hands and feet, but can also occur in other parts of the head, neck, chest, etc. EP usually occurs in middle age and there is no significant difference in incidence between men and women. The etiology of the disease is unknown, and the related factors mainly include genetics, trauma, solar exposure, radiation, chemoradiotherapy, pregnancy and HPV infection.7–9 Pathological examination is the main way to diagnose EP. Its pathological manifestations often need to be distinguished from BCC, seborrheic keratosis and other diseases. The basic pathological characteristics of typical poroma can be summarized as follows: (1) The tumor cells occur in the epidermis and extend into the dermis. They are mainly composed of cuboidal cells of the same size and shape, closely arranged, and deep basophilic nuclear and intercellular bridges can be seen; (2) The boundary between tumor cells and surrounding tissue is clear, but the surrounding cells of tumor body are not arranged in a palisade; (3) A narrow lumen or cystoid structure can be seen in the tumor strip, lined with a PAS-positive or EMA and CEA positive sheath and a layer of lumen cells;10–12 (4) Characteristic tumor cells contain a large amount of glycogen, resulting in cytoplasmic clarity, but usually uneven distribution; (5) Sometimes focal necrosis can be seen within the tumor; (6) In general, melanocytes and melanin particles are not seen in the tumor, but occasionally, poroma can be pigmented.13
BCC is the most common cutaneous malignancy. It often occurs on the face, hands and feet and other sun-exposed parts.14 BCC can usually occur on the basis of certain skin lesions such as solar keratosis, chronic ulcers, chronic infections, scar tissue and so on. BCC can be classified into various types in clinical pathology, including nodular/ulcerative type, pigmented type, superficial type, morpheaform type, infundibulocystic type and etcetera. Among them, nodular type is the most common, but there may also be multiple subtypes of histopathological manifestations in the same lesion.15 The cells in BCC have the potential to differentiate into the epidermis or appendages. Some studies believe that BCC can show a variety of specific cell lineage differentiation features, including keratotic BCC, infundibulocystic BCC, pleomorphic BCC, BCC with sebaceous differentiation, BCC with eccrine differentiation, the fibroepithelioma of Pinkus and so on.14 It has been suggested that about 1% of nodular BCC may be characterized by typical eccrine differentiation. This type of BCC is mainly manifested in the centralized distribution of cuboidal epithelial tubules in the eosinophilic Stratum corneum in the typical basaloid tumor cell aggregates, and the immunohistochemical staining of CEA and EMA can be seen in the eosinophilic Stratum corneum.16,17 (Immunohistochemical staining for EMA and CEA is a useful tool for the diagnosis of skin appendage tumors).2 In this case, we can see a large area of cuboidal poroma cells closely arranged and of the same size, surrounded by a small number of tumor clusters composed of basal-like cells. Therefore, after the consultation, we believe that this case is not consistent with eccrine differentiation in BCC. However, whether there is an internal relationship between the pathogenesis of EP and BCC in this case still needs further study.
In order to further clarify the pathological diagnosis of the lesion, we also developed some immunohistochemical staining to distinguish BCC, porocarcinoma, and trichoepithelioma (TE). BerEP4 is a monoclonal antibody with high specific expression in BCC, which can detect specific epithelial glycoprotein adhesion molecules (EpCAM) on BCC cells.18 Although it has been reported that focal positive expression of BerEP4 can also be found in some porocarcinoma, they usually lack peripheral palisading, which is not consistent with our case.19 The Bcl-2 protein encoded by the Bcl-2 gene prevents the release of cytochrome c from mitochondria into the cytoplasm, thereby inhibiting apoptosis. BCL-2 is diffusely positive in most BCC, but in TE it is mainly positive in the outermost epithelial cells.20 CD10 is a marker of germinal central cells and their derived lymphomas. CD10 was positive for basal-like cells in BCC and negative for peripheral stromal cells, but the opposite was true in TE, which was positive for peripheral stromal cells and negative for basal-like cells.21 CK7 is present in many glandular and transitional epithelium. Studies have shown that CK7 is highly expressed in BCC at non-sun exposed sites, while it is low or even negative expressed in BCC at long-term sun exposure. In this case, CK7 also showed low positive expression in the scalp lesions.22 Studies have shown that AR is expressed in many skin tumors, with about 50–60% of BCC cases expressing AR. In contrast, AR is barely expressed in mature hair follicle, epidermal, or benign hair follicle tumors such as TE. But in this case, we observed that AR was negative.21,23
Conclusion
In summary, we report a rare case of eccrine poroma with concurrent basal cell carcinoma. This case also demonstrates the importance of biopsy of all lesions to avoid missing a malignant tumor and affecting the patient’s treatment and prognosis.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon request.
Ethics Statement
The study protocol was approved by the ethical committees of Sinopharm Dongfeng General Hospital.
Consent Statement
A formal written consent was obtained for publication the case details and associated images from the patient.
Funding
The article had no funding source.
Disclosure
All authors stated no potential conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject of the manuscript.
References
1. Miller AC, Adjei S, Temiz LA, et al. Dermal duct tumor: a diagnostic dilemma. Dermatopathology. 2022;9(1):36–47. doi:10.3390/dermatopathology9010007
2. Wollina U, Castelli E, Rülke D. Immunohistochemistry of eccrine poroma and porocarcinoma–more than acrosyringeal tumors? Recent results in cancer research. Fortschritte Der Krebsforschung. 1995;1995:139303–139316.
3. Hasan A, Nafie K, Monazea K, et al. A rare case of recurrent eccrine poroma underlying gluteal abscess. Int J Surg Case Rep. 2020;75:7529–7531.
4. Cohen PR, Calame A. Multiple Skin Neoplasms at One Site (MUSK IN A NEST): a comprehensive review of basal cell carcinoma and benign or malignant “collision” tumors at the same cutaneous location. Clin Cosmet Investig Dermatol. 2020;13:731–741. doi:10.2147/CCID.S259324
5. AliFuat C, Andac A, Abdulkerim Y, et al. Nevus sebaceus with basal cell carcinoma, poroma, and verruca vulgaris. Indian J Pathol Microbiol. 2015;58(4):534. doi:10.4103/0377-4929.168885
6. Goldman P, Pinkus H, Rogin JR. Eccrine poroma; tumors exhibiting features of the epidermal sweat duct unit. AMA Arch Derm. 1956;74(5):511–521. doi:10.1001/archderm.1956.01550110055013
7. Sekine S, Kiyono T, Ryo E, et al. Recurrent YAP1-MAML2 and YAP1-NUTM1 fusions in poroma and porocarcinoma. J Clin Invest. 2019;129(9):3827–3832. doi:10.1172/JCI126185
8. Sidro-Sarto M, Guimerá-Martin-Neda F, Perez-Robayna N, et al. Eccrine poroma arising in chronic radiation dermatitis. J Eur Acad Dermatol Venereol. 2008;22(12):1517–1519. doi:10.1111/j.1468-3083.2008.02695.x
9. Nemoto I, Akiyama M, Aoyagi S, Nomura T, Shimizu H. Eccrine porocarcinoma and eccrine poroma arising in a scar. Br J Dermatol. 2004;150(6):1232–1233. doi:10.1111/j.1365-2133.2004.05997.x
10. Albert B, Mauricio G, Llúcia A, et al. The challenging diagnosis of eccrine poromas. J Am Acad Dermatol. 2016;74(6):e113–e115. doi:10.1016/j.jaad.2015.11.046
11. Robson A, Greene J, Ansari N, et al. Eccrine Porocarcinoma (Malignant Eccrine Poroma). Am J Surg Pathol. 2001;25(6):710–720. doi:10.1097/00000478-200106000-00002
12. Watanabe S, Mogi S, Ichikawa E, et al. Immunohistochemical analysis of keratin distribution in eccrine poroma. Am J Pathol. 1993;142(1):231–239.
13. Corrêa AMDA, Ré LMRD, Bartoli MLD, et al. Pigmented eccrine poroma in an atypical location. An Bras Dermatol. 2022;97(5):624–627. doi:10.1016/j.abd.2021.10.006
14. Crowson AN. Basal cell carcinoma: biology, morphology and clinical implications. Mod Pathol. 2006;19:S127–S147.
15. Tanese K. Diagnosis and management of basal cell carcinoma. Curr Treat Options Oncol. 2019;20(2). doi:10.1007/s11864-019-0610-0
16. Hanke CW, Temofeew RK. Basal cell carcinoma with eccrine differentiation (Eccrine Epithelioma). J Dermatol Surg Oncol. 1986;12(8):820–824. doi:10.1111/j.1524-4725.1986.tb01988.x
17. Misago N, Satoh T, Narisawa Y. Basal cell carcinoma with ductal and glandular differentiation: a clinicopathological and immunohistochemical study of 10 cases. Euro J Dermatol. 2004;14(6):383–387.
18. Ansai SI, Takayama R, Kimura T, Kawana S. Ber-EP4 is a useful marker for follicular germinative cell differentiation of cutaneous epithelial neoplasms. J Dermatol. 2012;39(8):688–692. doi:10.1111/j.1346-8138.2011.01494.x
19. Afshar M, Deroide F, Robson A. BerEP4 is widely expressed in tumors of the sweat apparatus: a source of potential diagnostic error. J Cutan Pathol. 2012;40(2):259–264. doi:10.1111/cup.12043
20. Iljin A, Stasikowska-Kanicka O, Zieliński T, et al. Immunoexpression of Bmi-1, CK15, Bcl-2 in different types of basal cell carcinomas. Adv DermatolAllergol. 2022;39(5):980–985. doi:10.5114/ada.2022.120888
21. Astarci HM, Gurbuz GA, Sengul D, et al. Significance of androgen receptor and CD10 expression in cutaneous basal cell carcinoma and trichoepithelioma. Oncol Lett. 2015;10(6):3466–3470. doi:10.3892/ol.2015.3804
22. García-de-la-Fuente MR, Santacana M, Valls J, et al. Cytokeratin profile of basal cell carcinomas according to the degree of sun exposure and to the anatomical localization. Am J Dermatopathol. 2018;40(5):342–348. doi:10.1097/DAD.0000000000001042
23. Evangelista MTP, North JP. Comparative analysis of cytokeratin 15, TDAG51, cytokeratin 20 and androgen receptor in sclerosing adnexal neoplasms and variants of basal cell carcinoma. J Cutan Pathol. 2015;42(11):824–831. doi:10.1111/cup.12546