Cardiovascular
Edwards gains CE mark approval for new tricuspid valve replacement device
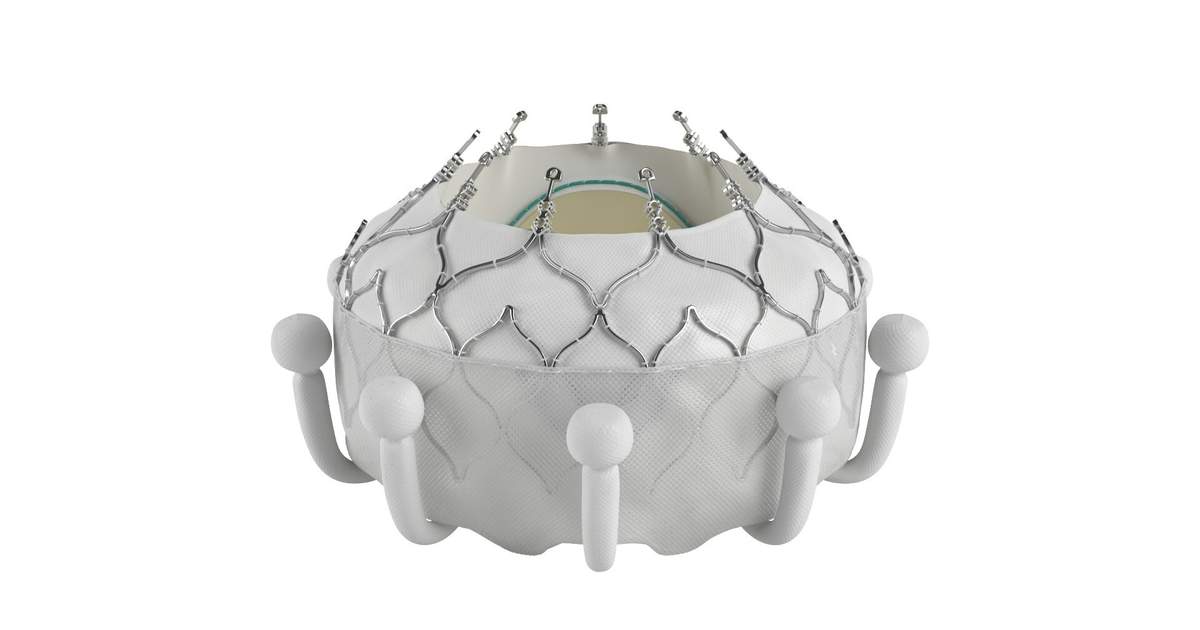
Edwards has been evaluating the safety and effectiveness of the Evoque system for quite some time. In November 2022, the company shared one-year data from the TRISCEND study that linked the system to a survival rate of 90.1% and sustained TR reduction in 97.6% of patients. Now, the company is preparing to share findings from the TRISCEND II study at the upcoming Transcatheter Cardiovascular Therapeutics (TCT) 2023 meeting in San Francisco.
“The Evoque system is able to fully replace the tricuspid valve, virtually eliminating tricuspid regurgitation in a wide range of anatomies,” cardiologist Philipp Lurz, MD, PhD, director of cardiology with the University of Mainz in Germany and European principal investigator for the TRISCEND II study, said in the same prepared statement. “The significant improvements in patients’ quality-of-life are remarkable, now offering a therapy to many patients who previously had no treatment options.”
The Evoque TTVR system is one of many medical devices currently being evaluated for the repair and replacement of a patient’s tricuspid valve. While it has not yet been approved by the U.S. Food and Drug Administration (FDA), receiving CE mark approval ahead of TCT 2023 suggests Edwards is gaining significant momentum and the Evoque system could potentially be the first FDA-approved TTVR system on the market.