Cancer and neoplasms
ESMO 2023: New Therapeutic Targets in Advanced Renal Cell Carinoma and Data from Sequencing Trials
(UroToday.com) The 2023 ESMO annual meeting included a session on optimizing overall survival in advanced renal cancer, featuring a presentation by Dr. Cristina Suarez Rodriguez discussing data from sequencing trials and new therapeutic targets. Dr. Suarez Rodriguez notes that there are several important definitions with regard to patterns of disease response to immune checkpoint inhibitors.
According to the Society of Immunotherapy of Cancer, primary/constitutive resistance is defined as progressive disease as best response or stable disease < 6 months. This requires a minimal duration of treatment of 6 weeks and confirmation of progressive disease with a second CT scan at 4 weeks. For adjuvant treatment, primary/constitutive resistance is a progressive disease in the 12 weeks after cessation of adjuvant treatment with confirmatory biopsy. For combination with targeted agents, primary/constitutive resistance is defined as progressive disease as best response or stable disease <6 months, a duration of 8-12 weeks, and does not need confirmation with a second CT scan.
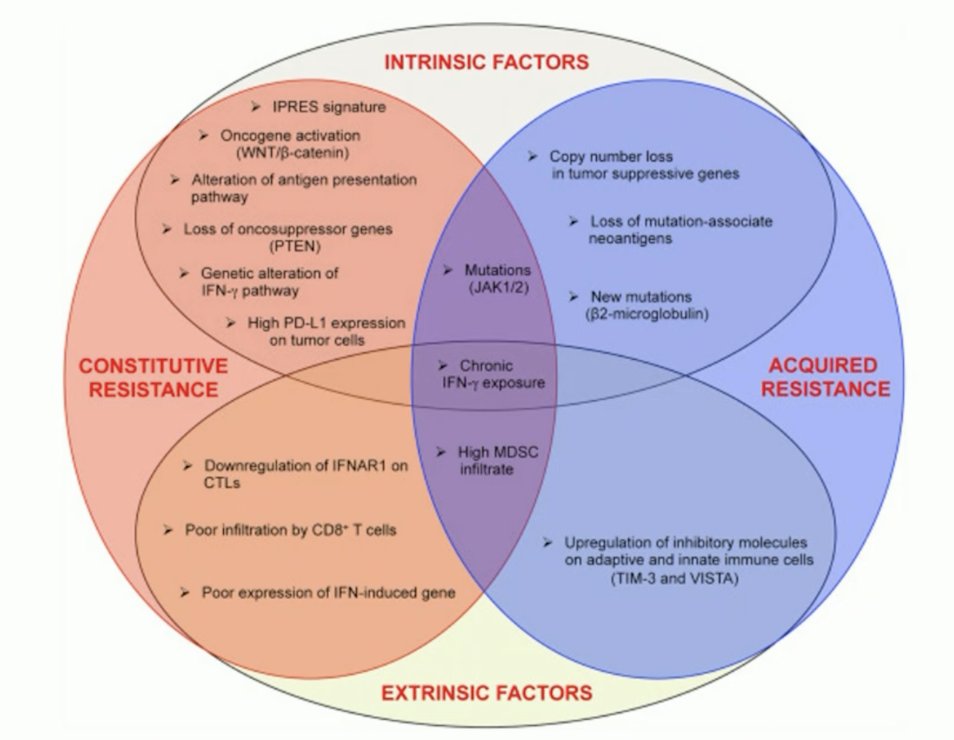
With regards to secondary/acquired resistance, this is defined as complete response, partial response, or stable disease lasting more than 6 months. This requires that the immunotherapy has been maintained for at least 6 months and a confirmatory scan is obtained after 4 weeks. Patients achieving partial or complete response lasting 6 months, drug exposure of 6 months, and does not require confirmation with a second CT scan. Indeed, there are many factors to consider when analyzing response and resistance to immune checkpoint inhibitors:
Based on the cancer immunity cycle, there are several treatment strategies to prevent and revert resistance:
Dr. Suarez Rodriguez notes that there are some important considerations when thinking about resistance:
- With the advent of first line IO-based combinations, the therapeutic sequence in metastatic RCC has changed
- After IO + IO, patients progress to “one mechanism of action”
- After IO + VEGF TKI, patients are progressing to “two mechanisms of action”
- The guidelines recommend a TKI in the second line if one has not been used before
As follows are TKI outcomes after IO combination therapies, with objective response rates of 20-54% and PFS rates of 6-12 months:
According to data from the IMDC database, starting with an IO + IO or IO + TKI combination therapy does not necessarily make a difference in outcomes when considering the sequencing of treatment:1
With regards to sequencing nivolumab to ipilimumab, the HCRN GU16-260, TITAN-RCC, OMNIVORE-RCC, and FRACTION trials have the following objective response rate outcomes:
For nivolumab + ipilimumab after IO, there are 7 studies, with 310 patients, and a reported objective response rate of 14%, and a median PFS of 3.7-5.5 months.
Dr. Suarez Rodriguez then discussed the recently published CONTACT-03 trial,2 which assessed atezolizumab plus cabozantinib versus cabozantinib monotherapy for patients with RCC after progression with previous immune checkpoint inhibitor treatment:
After a median follow-up of 15.2 months, the median progression-free survival was 10.6 months (95% CI 9.8-12.3) with atezolizumab + cabozantinib and 10.8 months (95% CI 10.0-12.5) with cabozantinib (HR 1.03, 95% CI 0.83-1.28):
The median overall survival was 25.7 months (95% CI 21.5-not evaluable) with atezolizumab + cabozantinib and was not evaluable (21.1-not evaluable) with cabozantinib (HR 0.94, 95% CI 0.70-1.27):
An ongoing trial in this disease space is the TINIVO-2 phase 3 trial assessing nivolumab + tivozanib versus tivozanib alone:
For the remainder of her presentation, Dr. Suarez Rodriguez discussed several ways that we can potentially optimize therapeutic sequence with new drugs:
- Alternative proangiogenic pathways
- Immunotherapies/enhancing IO
- Metabolic pathways
- PARP inhibitors
Alternative proangiogenic pathways
The mechanistic work delineating HIF-2alpha inhibition over the last several years led to the NCT03401788 trial assessing belzutifan in patients with VHL.3 This phase 2 open-label trial investigated the efficacy and safety of the belzutifan administered orally at a dose of 120 mg daily, in patients with renal cell carcinoma associated with VHL disease. The primary end point was objective response (complete or partial response) and other assessments included responses to belzutifan in patients with non–renal cell carcinoma neoplasms and the safety of belzutifan. Over a median follow-up of 21.8 months (range, 20.2 to 30.1 months), the percentage of patients with RCC who had an objective response was 49% (95% CI, 36 to 62). There were no complete responses, but 49% of patients had partial response, 49% had stable disease, and only 3% of patients had progressive disease. Remarkably, a reduction in the sum of all target lesion diameters was observed in 56 patients (92%):
Most patients had growing tumors before treatment, followed by an observed reduction in the sum of the largest tumor diameters after treatment began. The median time to response was 8.3 months, PFS at 24 months was 96%, 89% of patients were still on therapy at the time of data analysis, and median duration was not reached. Based on the results of this trial, the FDA approved belzutifan for cancers associated with VHL disease on August 13, 2021.
Belzutifan + cabozantinib phase 2 data was presented at ESMO 2022. Cohort 2 comprised patients who received prior immunotherapy and then subsequently belzutifan + cabozantinib. These patients had an objective response rate of 31%, PFS of 13.8 months, and OS of 24.1 months. The following clinical trials are also incorporating belzutifan:
Of note, Merck announced that the phase 3 LITESPARK-005 trial (belzutifan vs everolimus) met its primary endpoint of PFS in previously treated patients with advanced RCC.
Several additional potential treatment options relating to alternative proangiogenic pathways that Dr. Suarez Rodriguez briefly mentioned include: sitravatinib, XL-092, carbonic anhydrase IX, and 89Zr-girentuximab.
Immunotherapies/Enhancing IO
The first agent Dr. Suarez Rodriguez discussed was MEDI5752, which is a monovalent bispecific antibody targeting PD-1 and CTLA-4. This treatment has demonstrated objective response rates in the first line setting of 58.2% and in the second line setting of 21.4%. Second, NKTR-214 (bempegaldesleukin) + nivolumab was compared to investigator’s choice of sunitinib or cabozantinib in the phase 3 PIVOT-09 trial among treatment-naïve metastatic or advanced RCC, however this combination therapy did not improve outcomes compared to investigator’s choice of TKI. Third, CBM-588 + nivolumab/ipilimumab has been assessed in a phase 1 trial. The rationale for CBM-588 is that this is a live bacterial product with retrospective data suggesting it may augment the activity of checkpoint inhibitors. This may occur via immunomodulation of the gut and/or alteration of gut microbiome composition. The trial schema is as follows:4
This trial found that PFS was significantly longer in patients receiving nivolumab-ipilimumab with CBM588 than without (12.7 months versus 2.5 months, HR 0.15, 95% CI 0.05-0.47). Although not statistically significant, the response rate was also higher in patients receiving CBM588 (58% versus 20%, p = 0.06):
Metabolic Pathways
Telaglenastat has been assessed in the ENTRATA and CANTATA trials with median PFS of telaglenastat + everolimus of 3.8 months and telaglenastat + cabozantinib of 9.2 months:
Ciforadenant is an adenosine A2a receptor antagonist, which demonstrated a median PFS of 4.1 months alone and 5.8 months in combination with atezolizumab. Additionally, 90% of patients were alive at 25 months in the combination arm:
PARP Inhibitors
The rationale for PARP inhibitors in RCC is that hypoxia drives the development of genomic instability via downregulation of DNA repair gene expression. Furthermore, DNA repair genes are downregulated by hypoxic stress and decreased in VHL-deficient renal cancer cells, and this gene repression is associated with impaired double-strand break repair in VHL-deficient cells. Thus, VHL deficiency confers increased sensitivity to PARP inhibitors.
There is also rationale to possibly combine IO with PARP inhibitors in RCC. In metastatic RCC, DDR mutations are present in ~19% of tumors, with the most common alterations being CHEK2 and ATM. DDR mutation status was associated with superior OS for patients with IO treatment (HR 0.41) and there was no association with VEGF-TKI treatment. Of note, there are several ongoing PARP inhibitor trials for RCC:
Dr. Suarez Rodriguez concluded her presentation discussing data from sequencing trials and new therapeutic targets with the following take-home points:
- With the advent of first-line combinations, the second-line scenario has changed and data from phase 3 studies is awaited
- After IO + IO or IO + TKI, a TKI has the strongest evidence as of now
- Ongoing trials may provide more information about the role of new targets
- Belzutifan can be a new player in the sequencing of treatment for RCC
- In this population, participating in clinical trials is highly encouraged
Presented by: Cristina Suarez Rodriguez, MD, PhD, Vall D’Hebron University Hospital, Barcelona, Spain
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2023 European Society of Medical Oncology (ESMO) Annual Meeting, Madrid, Spain, Fri, Oct 20 – Tues, Oct 24, 2023.
References:
- Dudani S, Graham J, Wells JC, et al. First-line Immuno-Oncology Combination Therapies in Metastatic Renal-cell Carcinoma: Results from the International Metastatic Renal-Cell Carcinoma Database Consortium. Eur Urol. 2019 Dec;76(6):861-867.
- Pal SK, Albiges L, Tomczak P, et al. Atezolizumab plus cabozantinib versus cabozantinib monotherapy for patients with renal cell carcinoma after progression with previous immune checkpoint inhibitor treatment (CONTACT-03): A multicenter, randomized, open-labl, phase 3 trial. Lancet 5 June 2023 [Epub ahead of print].
- Jonasch E, Donskov F, Iliopoulos O, et al. Belzutifan for Renal Cell Carcinoma in von Hippel-Lindau Disease. N Engl J Med. 2021;385:2036-2046.
- Dizman N, Meza L, Bergerot P, et al. Nivolumab plus ipilimumab with or without live bacterial supplementation in metastatic renal cell carcinoma: A randomized phase 1 trial. Nat Med. 2022 Apr;28(4):704-712.