Cancer and neoplasms
Recurrent chemotherapy treated IDCT
1Department of Nephrology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, People’s Republic of China; 2Department of Hematology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, People’s Republic of China
Correspondence: Jing Xiong, Department of Nephrology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, 430022, People’s Republic of China, Tel +86 18963953980, Email [email protected]
Abstract: Indeterminate dendritic cell tumor (IDCT) is an extremely uncommon histiocytic and dendritic neoplasms subtype that presents as single or multiple papules. There is currently no standard method for diagnosis and treatment, and the selection of therapeutic approaches is mainly based on successful examples of folk medicine. We describe a case of a pathology diagnosed indeterminate dendritic cell tumor, which shows the presence of CD1a, S100, and CD68, but lack langerin. She was treated with multi-chemotherapy regimens used to treat lymphoma and gained good results short term but was easy to recur. In addition, we reviewed the literature on the effectiveness and safety of chemotherapy in IDCT patients.
Keywords: indeterminate cell histiocytosis, indeterminate dendritic cell tumor, chemotherapy, recurrent, S100 atypical
Introduction
Indeterminate dendritic cell tumor (IDCT), also called indeterminate cell histiocytosis (ICH), was first reported in 1985 by Wood et al.1 It can be categorized under the “L” group of histiocytosis as per the 2016 Histiocyte Society classification. Pathologically, IDCT exhibits CD1a positivity like Langerhans cell histiocytosis; however, negativity for langerin (CD207) allows for a distinctive differentiation from Langerhans cell histiocytosis.2 According to both the 5th edition of the World Health Organization Classification of Haematolymphoid Tumours,3 and the International Consensus Classification of Mature Lymphoid Neoplasms,4 IDCT belongs to the subgroup of dendritic cell and histiocytic neoplasms.
Its pathophysiology, etiology, and prognostic features are poorly understood due to its rarity. Here we report a multi-chemotherapy treated IDCT patient. Reported IDCT cases using chemotherapy regimens as treatment are reviewed. As far as we know, these kinds of therapies in our patient have never been used in any other Chinese IDCT patient before.
Case Presentation
A 47-year-old female patient in otherwise good health came to the hospital with a 2-month history of diffuse papules with slight pruritus. The papules started on the left forearm without pain, ululation, and purulent discharge and then gradually spread to the trunk, extremities, face, and scalp (Figure 1). Before referral, the patient was diagnosed as blastic plasmacytoid dendritic cell neoplasm (BPDCN), the patient came to our hospital to seek a second opinion.
|
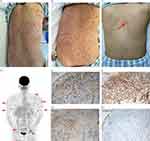
Figure 1 (A–C) Skin condition before and after treatment. Upon admission, multiple yellowish-red or reddish-brown papules and nodules almost spread throughout the body, with clear boundaries and partial fusion. The back area was the most severe (A). After the completion of 4 courses of CHOP+ lenalidomide, the rash subsided significantly, with significant pigmentation on the back (B). Two courses of chemotherapy were completed after recurrence, and the rash almost completely subsided with only a small amount of pigmentation on the back (red arrow, biopsy scar after recurrence) (C). (D) [18F] FDG-PET/CT showed considerable metabolic increases in the skin (the red arrows point to some areas exhibiting heightened metabolic activity). Areas including the upper limbs (upper arm), lower neck, body (back), and the skin/subcutaneous area of the upper thighs of both sides within the detection range, and some of them were accompanied by small nodular shadows. The possibility of malignant tumor lesions was considered. Immunohistochemical studies demonstrated that the histiocytes were positive for CD1a (E), CD68 (F) and S-100 (G) and negative for Langerin (H). |
Pathological consultation in our hospital showed that: S100 (partially +), CD1a (+), Langerin (-), CD4 (+), CD56 (partially +), CD68 (+), CD163 (-), Cyclin D1 (+), Ki67 (LI: 20%), CD3 (-), CD20 (-), CD200 (-), TdT (-), BRAF (-), TCF4 (-), ALK (-), SOX10 (-), according to the clinical manifestations, histopathology, and immunohistochemistry, the diagnosis of indeterminate dendritic cell tumor (IDCT) was made.
To exclude other malignant tumors, the patient underwent PET-CT (Figure 1), and the possibility of malignant tumor lesions was considered. Bone marrow aspiration and biopsy parallel with karyotype analysis (46, XX5), immunophenotyping, and TCR gene testing showed no results of Hematopoietic malignancies. The patient was treated with a course of CHOP plus lenalidomide every three weeks for four courses. The patient’s rash improved significantly after the first course of treatment. After completing four courses, the lesions subsided significantly, leaving only pigmentation on the back.
In the next six months, the patient continued to be in remission. Until May 2022, when the rash recurred, the skin biopsy of the back lesion was performed again, and the immunochemistry revealed no significant changes except for S100 compared with the previous one (Figure 1). At the same time, bone marrow aspiration was performed again to exclude malignancy. No notable findings were found. We continued the prior chemotherapy regimen, except for thalidomide instead of lenalidomide, which the patient continued to respond to, but the rash subsided more slowly than before, and she is prone to relapse once drug cessations. The patient did not undergo genetic testing due to economic constraints. She is now treated by the local hospital with another chemotherapy, Oxaliplatin, Gemcitabine, and Betamethasone. According to the latest follow-up phone call, the rash was fading; however, she experienced severe bone marrow suppression midway through the process. Figure 2 shows the timeline of our case.
|
Figure 2 The timeline of this case report. Abbreviations: CTX, Cyclophosphamide; DOX, doxorubicin; VDS, Vindesine; DEX, dexamethasone; LOHP, Oxaliplatin; GEM, Gemcitabine; NA, not available. |
Literature Review
IDCT belongs to a subgroup of dendritic cell and histiocytic neoplasms,3,4 presenting histological, ultrastructural, and immunophenotypic characteristics of Langerhans cells but missing Birbeck granules. The pathogenesis and etiology of this condition remain unclear.
Epidemiology
Up to now, only roughly 100 cases have been documented in the literature. In China, just approximately ten instances have been reported. IDCT is most frequent in adults and has no gender preference, but it has also been recorded in children.6
Pathogenesis
Because of the rarity of IDCT, its pathogenesis is not clear. It has been observed that an itchy rash appears after being bitten by a mosquito or a scabies mite and is subsequently identified as IDCT. Because of this, it is speculated that the reactive inflammatory process is the mechanism of IDCT. Genetic factors are also reported to be involved in the pathogenesis of IDCT but remain obscure. Brown et al7 detected ETV3-NCOA2 gene mutation in all three of their IDCT patients, while Davick et al8 found otherwise in their two. Thurner et al9 successfully kept an IDCT patient with a BRAF V600E mutation in metabolic remission for 23 months using BRAF/MEK Inhibition. New concepts for clinical diagnosis and therapy have emerged due to the identification of these genes. Due to budgetary issues, our patients cannot obtain gene-related testing.
Clinical Presentations
Regarding clinical presentations, most patients exhibit single or numerous papules or nodules confined to the skin. A tiny percentage of individuals experience progression and fusion of lesions, and some of these patients may exhibit unique lesions like leonine facies or pityriasis Rosacea. It has been reported that IDCT can affect extra-skin areas such as the conjunctiva, spine, spleen, and pancreas and that the disease is associated with malignant tumors, particularly hematological tumors10,11 (myeloid leukemia, follicular lymphoma, etc.), suggesting that we need to run through the examinations of blood and other systems. In our case, papules spread quickly within two months, affecting practically the entire body, but without Extracutaneous involvement.
Diagnosis
The diagnosis of IDCT relies mainly on pathological examination. Our patient was firstly diagnosed with possible BPDCN in her local hospital. Like IDCT, BPDCN are often characterized with cutaneous lesions and can involve bone marrow, lymphonodus and internal organs in different degree, but BPDCN has a more aggressive clinical course and worse prognosis. The pathological consultation in our hospital do show the presence of CD4 and CD56. However, the presence of four or more markers including CD4, CD56, CD123, TCL1, TCF4, and CD303 is imperative for the accurate diagnosis of BPDCN, which our patient did not exhibit, can exclude the diagnosis of BPDCN. Another important differential diagnosis is Langerhans cell histiocytosis (LCH). IDCT could be distinguished from LCH by the absence of langerin expression and Birbeck granules.
Treatment
Various treatments, either alone or in combination, have yielded promising results in IDCT patients, including total skin electron beam therapy, narrow-band UVB therapy, plus ultraviolet A therapy, Topical 5-fluorouracil, topical corticosteroids, pravastatin, bath-psoralen, chemotherapies, thalidomide, and BRAF/MEK Inhibition (if BRAF V600E mutation exists),8 etc. Given the patient’s PET-CT results indicating increased metabolic activity in bilateral inguinal and axillary lymph nodes, coupled with literature suggesting a potential co-occurrence of lymphoma in IDCT cases, we opted for the CHOP regimen. The selection of lenalidomide was based on successful experiences with thalidomide-class drugs in the therapeutic management of IDCT, as identified through literature retrieval. During treatment, our patient developed bone marrow suppression and decreased blood cells, so the 3rd course of lenalidomide was only taken for seven days. The patient responded sensitively to this chemotherapy regimen, and the lesions improved significantly at the end of the first course, with only dorsal hyperpigmentation remaining after completing four courses. She maintained remission for about six months but relapsed.
We reviewed the current examples of IDCT with cutaneous symptoms treated with chemotherapy medications to assess the efficacy and safety of chemotherapy drugs1,5,11–30 (Table 1). We found that remission and relapse occurred in 8 out of 26 chemotherapy-treated patients reported to date.5,11,16,24,25 Among the reported cases, six of them achieved complete remission. The average follow-up period was 47 months (range: 10–114 months). Interestingly, half of the patients experienced a process of remission and subsequent relapse.20,22 Additionally, 6 out of 26 reported cases died, all of them are found to have haematological neoplasia after chemotherapy initiated.11,23 Although it has been reported that IDCT is related to malignant tumors, the carcinogenic effect of chemotherapy drugs themselves cannot be ruled out.
|
Table 1 Reported Cases of IDCT with Cutaneous Symptoms Treated with Chemotherapy Medications |
Conclusion
In conclusion, we report a case of IDCT presenting with multiple papules, markedly improving after treatment but recently recurred. At the same time, the expression of S100 changed from partially positive to positive. I believe our case has contributed to understanding IDCT and the complex adjustment mechanism of S100 expression. Additionally, after analyzing chemotherapy treated IDCT patients, we suggest a more cautious chemotherapy medication selection. Long-term follow-up is required due to the link between lymphoproliferative illness and hematological malignancies.
Ethics Statement
The patients in this manuscript have given written informed consent to publication of their case details and any accompanying images. No institutional approval is required for the publication of this case.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by grants from the National Natural Science Foundation of China (No. 81770736).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Wood GS, Hu CH, Beckstead JH, Turner RR, Winkelmann RK. The indeterminate cell proliferative disorder: report of a case manifesting as an unusual cutaneous histiocytosis. J Dermatol Surg Oncol. 1985;11(11):1111–1119. doi:10.1111/j.1524-4725.1985.tb01399.x
2. Emile JF, Abla O, Fraitag S, et al. Revised classification of histiocytoses and neoplasms of the macrophage-dendritic cell lineages. Blood. 2016;127(22):2672–2681. doi:10.1182/blood-2016-01-690636
3. Khoury JD, Solary E, Abla O, et al. The 5th edition of the World Health Organization classification of haematolymphoid tumours: myeloid and histiocytic/dendritic neoplasms. Leukemia. 2022;36(7):1703–1719. doi:10.1038/s41375-022-01613-1
4. Campo E, Jaffe ES, Cook JR, et al. The international consensus classification of mature lymphoid neoplasms: a report from the clinical advisory committee. Blood. 2022;140(11):1229–1253. doi:10.1182/blood.2022015851
5. Fournier J, Ingraffea A, Pedvis-Leftick A. Successful treatment of indeterminate cell histiocytosis with low-dose methotrexate*. J Dermatol. 2011;38(9):937–939. doi:10.1111/j.1346-8138.2010.01148.x
6. Kong FW, Read J, Pitney L, Tang F, Davidson S, Wheller L. S100‐negative indeterminate cell histiocytosis: in an eight‐month‐old boy. Australas J Dermatol. 2020;62(1):e124–e127. doi:10.1111/ajd.13390
7. Brown RA, Kwong BY, McCalmont TH, et al. ETV3-NCOA2 in indeterminate cell histiocytosis: clonal translocation supports sui generis. Blood. 2015;126(20):2344–2345. doi:10.1182/blood-2015-07-655530
8. Davick JJ, Kim J, Wick MR, Gru AA. Indeterminate dendritic cell tumor: a report of two new cases lacking the ETV3-NCOA2 translocation and a literature review. Am J Dermatopathol. 2018;40(10):736–748. doi:10.1097/DAD.0000000000001191
9. Thurner L, Bewarder M, Rosar F, et al. Indeterminate dendritic cell tumor with persistent complete metabolic response to BRAF/MEK inhibition. HemaSphere. 2021;Vol. 5(1). doi:10.1097/hs9.0000000000000511
10. Loghavi S, Curry JL, Garcia-Manero G, et al. Chronic myelomonocytic leukemia masquerading as cutaneous indeterminate dendritic cell tumor: expanding the spectrum of skin lesions in chronic myelomonocytic leukemia. J Cutan Pathol. 2017;44(12):1075–1079. doi:10.1111/cup.13039
11. Vener C, Soligo D, Berti E, et al. Indeterminate cell histiocytosis in association with later occurrence of acute myeloblastic leukaemia. Br J Dermatol. 2007;156(6):1357–1361. doi:10.1111/j.1365-2133.2007.07880.x
12. Xia DM, Zhou QT, Wang L. Typical leonine facies in a patient with indeterminate dendritic cell tumor. Int J Dermatol. 2022;62(1):128–129. doi:10.1111/ijd.16106
13. Lie E, Jedrych J, Sweren R, Kerns ML. Generalized indeterminate cell histiocytosis successfully treated with methotrexate. JAAD Case Rep. 2022;25:93–96. doi:10.1016/j.jdcr.2022.05.027
14. Belina ME, Kwock JT, Al-Rohil R, Fresco A. An atypical myelomonocytic cell infiltrate: use of next-generation sequencing to diagnose indeterminate cell histiocytosis. Am J Dermatopathol. 2022;44(7):529–531. doi:10.1097/dad.0000000000002167
15. Fischer AS, Zaladonis AG, Subrt P, Tschen J, Hsu S. Indeterminate cell histiocytosis mimicking rosacea. Cureus. 2021;13(1):e12850. doi:10.7759/cureus.12850
16. Zhou N, Ge Y, Fang K, et al. BRAF wild-type recurrent indeterminate dendritic cell tumour presenting with leonine facies. J Eur Acad Dermatol Venereol. 2020;34(5):e230–e31. doi:10.1111/jdv.16172
17. Chen C, Nguyen GH, Zeng YP. Indeterminate cell histiocytosis presenting with leonine facies. Acta Derm Venereol. 2018;98(4):463–464. doi:10.2340/00015555-2866
18. Xu XL, Bu WB, Zong WK, Sun JF. Indeterminate cell histiocytosis: a case series and review of the literature. Eur J Dermatol. 2017;27(5):559–561. doi:10.1684/ejd.2017.3121
19. Horna P, Shao H, Idrees A, Glass LF, Torres-Cabala CA. Indeterminate dendritic cell neoplasm of the skin: a 2-case report and review of the literature. J Cutan Pathol. 2017;44(11):958–963. doi:10.1111/cup.13017
20. Tóth B, Katona M, Hársing J, Szepesi A, Kárpáti S. Indeterminate cell histiocytosis in a pediatric patient: successful treatment with thalidomide. Pathol Oncol Res. 2012;18(2):535–538. doi:10.1007/s12253-011-9405-8
21. Bettington A, Lai JK, Kennedy C. Indeterminate dendritic cell tumour presenting in a patient with follicular lymphoma. Pathology. 2011;43(4):372–375. doi:10.1097/PAT.0b013e32834685b7
22. Yin R, Zheng WJ, Yang XC, Hao F. Recurrent generalized indeterminate cell histiocytosis: a case report. J Am Acad Dermatol. 2010;63(1):e3–5. doi:10.1016/j.jaad.2009.10.010
23. Ventura F, Pereira T, da Luz Duarte M, Marques H, Pardal F, Brito C. Indeterminate cell histiocytosis in association with acute myeloid leukemia. Dermatol Res Pract. 2010;2010:569345. doi:10.1155/2010/569345
24. Cheuk W, Cheung FY, Lee KC, Chan JK. Cutaneous indeterminate dendritic cell tumor with a protracted relapsing clinical course. Am J Surg Pathol. 2009;33(8):1261–1263. doi:10.1097/PAS.0b013e3181a0cd38
25. Caputo R, Marzano AV, Passoni E, Bellinvia M. Chemotherapeutic experience in indeterminate cell histiocytosis. Br J Dermatol. 2005;153(1):206–207. doi:10.1111/j.1365-2133.2005.06644.x
26. Kolde G, Brocker EB. Multiple skin tumors of indeterminate cells in an adult. J Am Acad Dermatol. 1986;15(4 Pt 1):591–597. doi:10.1016/s0190-9622(86)70209-0
27. Segal GH, Mesa MV, Fishleder AJ, et al. Precursor Langerhans cell histiocytosis. An unusual histiocytic proliferation in a patient with persistent non-Hodgkin lymphoma and terminal acute monocytic leukemia. Cancer. 1992;70(2):547–553. doi:10.1002/1097-0142(19920715)70:23.0.co;2-z
28. Flores‐Stadler EM, Gonzalez‐Crussi F, Greene M, Thangavelu M, Kletzel M, Chou PM. Indeterminate‐cell histiocytosis: immunophenotypic and cytogenetic findings in an infant. Med Pediatr Oncol. 1999;32(4):250–254. doi:10.1002/(sici)1096-911x(199904)32:43.0.Co;2-#
29. Rodriguez-Jurado R, Vidaurri-de la Cruz H, Duran-Mckinster C, Ruiz-Maldonado R. Indeterminate cell histiocytosis. clinical and pathologic study in a pediatric patient. Arch Pathol Lab Med. 2003;127(6):748–751. doi:10.5858/2003-127-748-ICH
30. Ozono S, Inada H, Nakagawa SI, et al. Juvenile myelomonocytic leukemia characterized by cutaneous lesion containing Langerhans cell histiocytosis-like cells. Int J Hematol. 2011;93(3):389–393. doi:10.1007/s12185-011-0787-x