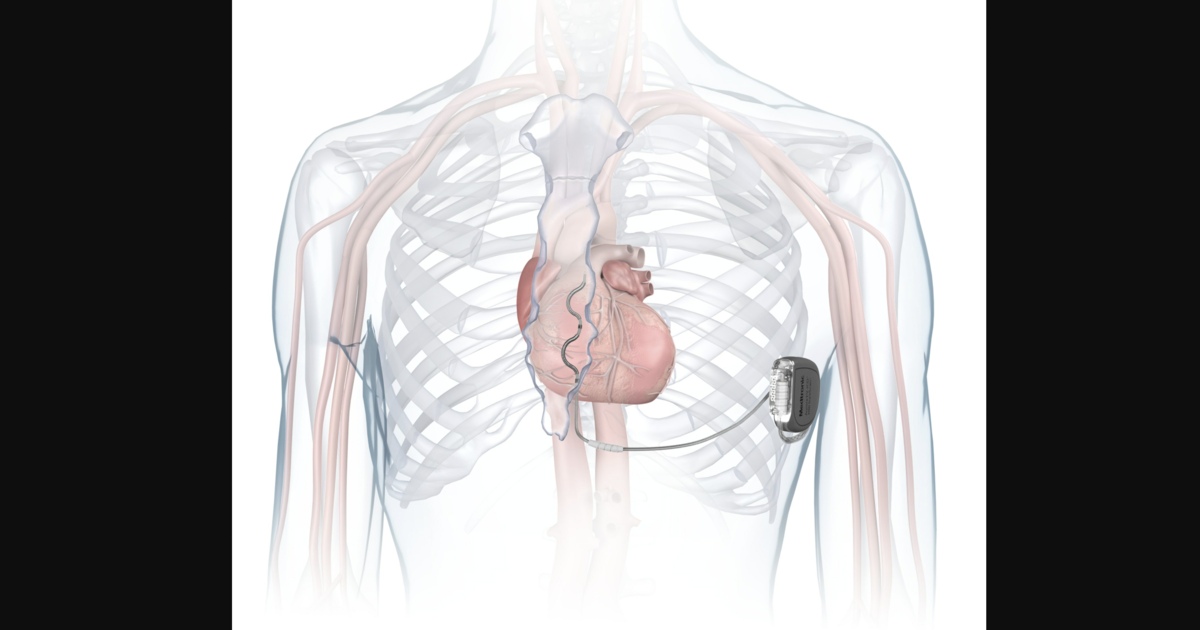
Cardiovascular
FDA approves Medtronic’s extravascular ICD for abnormal heart rhythms
“The Aurora EV-ICD system is a tremendous step forward in implantable defibrillator technology,” Bradley P. Knight, MD, medical director of electrophysiology at Northwestern Medicine Bluhm Cardiovascular Institute and a researcher with experience evaluating the newly approved device, said in a prepared statement. “Placing the leads outside of the heart, rather than inside the heart and veins, reduces the risk of long-term complications, ultimately allowing us to further evolve safe and effective ICD technology.”
“This FDA approval paves the way for patients to have a better overall experience with ICD therapy,” added Alan Cheng, MD, chief medical officer of Medtronic’s cardiac rhythm management business. “ICDs remain the gold standard for prevention of sudden cardiac death, and while the subcutaneous ICD avoids certain complications associated with transvenous defibrillators, it has limitations that may affect a patient’s comfort and quality-of-life.”
The Aurora EV-ICD MRI SureScan and Epsila EV MRI SureScan defibrillation leads previously gained CE mark approval back in February 2023. They are expected to be commercially available in the United States on a limited basis in the weeks ahead.