Cancer and neoplasms
Ovarian Clear Cell Carcinoma with Recurrent Endometriosis
1The Second Clinical College of Zhejiang Chinese Medical University, Zhejiang Chinese Medical University, Hangzhou, Zhejiang, People’s Republic of China; 2Department of Obstetrics and Gynecology, The Second Affiliated Hospital of Zhejiang Chinese Medical University (Xinhua Hospital of Zhejiang Province), Hangzhou, Zhejiang, People’s Republic of China
Introduction: Endometriosis-associated ovarian cancer (EAOC) is rare, occurring approximately in 1% of women with ovarian endometriosis. The main histological types are endometrioid adenocarcinoma and clear cell carcinoma (CCC), with the latter being the least common.
Case Presentation: In our hospital, we recently summarized two patients with ovarian clear cell carcinoma with similar characteristics. They all had endometriosis for a long time and had undergone ovarian cyst removal due to a chocolate cyst. Unfortunately, the cyst recurred after surgery, and the histological diagnosis was clear cell carcinoma. In case 1, the expression of P53 was found in the tumor by regular examination, and the stage was IIB. In Case 2, we found it in intraoperative freezing; the stage was IA. Case 1 has been treated with the TP regimen six times, and the survival period has reached one year. Case 2 had a survival period of 6 years after completing six TP regimen treatments. Clinicians should pay attention to patients with a long history of endometriosis and postoperative recurrence of ovarian cysts accompanied by serum CA-125 of more than 200U/mL. Imaging examination has a good prospect in diagnosing malignant transformation of endometriosis, especially PET-CT.
Conclusion: This case report highlights the risk factors related to the formation of ovarian clear cell carcinoma and suggests that the follow-up of patients with ovarian endometriosis is essential because of its recurrence characteristics. Radical surgery and postoperative platinum-containing chemotherapy are the primary treatment methods at present.
Keywords: gynecological tumor, endometriosis, malignant transformation, ovarian clear cell carcinoma, case report
Introduction
Endometriosis (EMs) is a hormone-dependent benign disease. Endometriosis and glands grow outside the uterine cavity. Ectopic endometrial tissue can invade any body part, mainly in the pelvic organs and parietal peritoneum. The proliferation, infiltration, and metastasis characteristics in EMs are similar to malignant tumors. EMs often occurs in women of childbearing age, with a malignant transformation rate as high as 0.7% ~ 2.5%.1 However, compared with EMs in the childbearing period, perimenopausal and postmenopausal women have an increased risk of malignant transformation.2 The most common site is the ovaries.3 Studies show the risk of ovarian endometriosis cyst malignant transformation into ovarian cancer is about 2% ~ 3%.4 The most common histological manifestations are endometrioid and clear cell carcinoma. EAOC is thought to develop from ovarian and endometrial cysts (ECs). Ovarian endometrioid carcinoma (OEC) is the most common type of EAOC, occurring in approximately 75% of cases. Clear cell carcinoma is an uncommon histologic subtype of ovarian carcinoma with poor response to Platinum-based chemotherapy agents at high stages. Compared with other histological subtypes of epithelial ovarian cancer, advanced CCC is usually associated with a worse prognosis because of its increased resistance to platinum-based chemotherapy.5 So, it has the worst prognosis and is often difficult to treat.5
The mechanism of endometriosis-associated malignancies is not well understood. Current studies have shown that inflammatory responses, hormonal imbalances, oxidative stress, mutations in ARID1A, PIK3CA, KRAS, or PTEN genes, loss of expression of mismatch repair enzymes, and microsatellite instability are involved. In EAOC, clear cell carcinoma has the worst prognosis and is often difficult to treat.
Case Presentation
Case 1
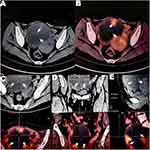
In February 2022, a 45-year-old woman was admitted to the outpatient department because her abdominal mass increased in the short term. The external hospital’s PET/CT (Figure 1) showed the right ovarian cystadenocarcinoma (12.3* 9.6 cm), and the nature was cystic-solid. The patient had ovarian cyst removal surgery seven years ago, combined with a gonadotropin-releasing hormone agonist (GnRH-A) six times after the operation. The cyst recurred after one year, and the following regular ultrasound showed that the mass gradually increased. In December 2021, the patient found a mixed echo in the right adnexal area, CA125 reached 254.9U/mL, and CA199 reached 58.25U/mL. In February 2022, the patient found that the abdominal mass was conspicuous when lying flat, and an ultrasound showed that the right adnexal area was a solid cystic mass.
|
Figure 1 PET-CT (Case 1) A pelvic cystic-solid mass was considered to originate from right ovarian cystadenocarcinoma. Notes: The cystic component was mainly cystic; the thickness of the cyst wall was uneven, multiple wall nodules were seen, and the uptake was increased (the arrow symbols). The maximum standardized uptake value (SUV) was 3.3. (A–D) shows the cyst cross-section in different axial planes, and (E) shows the cyst cross-section in the sagittal plane. Red, bright spots indicate the tracer color and suggest areas of abnormal metabolism. |
On admission, the laboratory examination showed CA125: 45.9U/mL. We can feel a lump up to 3 cm below the umbilicus in the patient’s abdomen. After fully explaining to the patient, they jointly decided to perform laparoscopic exploration on February 28, 2022. During the procedure, some intestines adhere to the abdominal wall. The appendages are attached to the four sides of the pelvic border. The right ovary was about 15 cm in diameter, with a smooth, gray-white, cystic color, and occupied the entire pelvis. The bottom of the mass has tightly adhered to the intestine, the posterior lobe of the right broad ligament, and the right uterosacral ligament; During the separation, the right ovarian mass ruptured, and the brown chocolate-like liquid flowed out, and Some of them showed carrion-like lesions. Send fast freezing, indicating (right) ovarian adenocarcinoma. On the posterior wall of the vagina, we see an internal heterogeneous nodule with a chocolate outflow of fluid.
During the operation, we converted laparoscopic surgery to open surgery. We performed a total hysterectomy, bilateral adnexectomy, paraaortic lymphadenectomy, pelvic lymph node dissection, omentectomy, appendectomy, and pelvic adhesiolysis. R0 resection was achieved. The peritoneal lavage fluid indicated no tumor cells. Hematoxylin and eosin staining (Figure 2) showed clear cell carcinoma (right ovary) without nerve and vascular invasion; (Right sacral ligament). Pathology in the right sacral ligament suggests a small amount of cancerous tissue in the ovary and fibrous tissue, and we did not find metastatic cancer in the rest. The immunohistochemical results were CK7+, P16+, EMA+, CA125+, P53 scattered+, CK20 -, ER -, PR -, WT-1 -, Ki6730%+. The postoperative diagnosis was stage IIB of ovarian clear cell carcinoma. After the operation, the patient received six times of combined chemotherapy of paclitaxel and platinum. The patient developed bone marrow suppression (leukopenia) and drug-induced liver damage during chemotherapy, which improved after symptomatic treatment. At present, no recurrence is found after 1-year follow-up. The patient’s history and current information about this treatment can be clearly shown in Figure 3.
|
Figure 2 Pathology (hematoxylin and eosin staining). Note: Case 1 (A and B); Case 2 (C and D). |
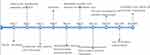 |
Figure 3 Timeline of the case presentation with relevant data. Note: Case 1. |
Case 2
In April 2017, a 54-year-old woman was hospitalized in our hospital after menopause for more than 20 years due to dysmenorrhea. Ultrasound in other hospitals suggested that adenomyosis should be considered first; Bilateral ovarian cystic mass: chocolate cyst may be. The patient underwent cystectomy abroad for a “chocolate cyst” 15 years ago and received GnRH-A six times after surgery. In February 2015, the local hospital ultrasound examination showed that the bilateral ovarian cystic mass size was about 54 * 36mm and 55 * 47mm.
On admission, the laboratory test CA125 was 48.8U/mL. A palpable mass was between the umbilicus and pubic bone. It is hard, poorly acted, and non-tender.
Laparoscopic exploration was performed on April 24, 2017. During the operation, we found dense adhesion between the intestine and the fundus, posterior wall and bilateral appendages. The left ovary had three cysts, with the largest diameter of about 10 centimeters. The inner fluid is chocolate-liquid-like. The diameter of the right ovary is about 4 centimeters. The inner fluid tightly adheres to the surrounding tissues. The rectum and uterus are completely closed. ASRM score is 128 points. The left ovarian cyst ruptured and flowed out 300mL of light coffee fluid during the separation and adhesion. The size of papillary tissue between the inner walls of the ovary was about 2 * 3cm, and the texture was fragile. The intraoperative freezing report: (left attachment) There was no obvious overlying epithelium in the cystic wall-like tissue. In addition, there was obvious hyperplasia of adenoid structure in the solid tissue, which was to be confirmed by routine and immunohistochemistry. The results of peritoneal lavage showed that the malignant cells were negative. Postoperative pathology showed that the left ovarian clear cell carcinoma was in stage IA. Immunohistochemical results: CK7+, CA125+, CK20 -, ER -, PR -, WT – 1 -, Ki67 15%+. Postoperative reexamination of CA125 is 217.1U/mL, considered to be caused by cyst rupture during operation. After the operation, six TP chemotherapy sessions were given after surgery without supplementary surgical treatment, and the process was smooth. Now the recovery is good six years after the operation, without recurrence.
Discussion
The overall malignant transformation rate of endometriosis is about 1.0%. Studies have shown regional differences in the incidence of different histological types. In the United States, the prevalence of EM-induced clear cell carcinoma is only 3.1% – 11.1%, while in East Asia, the majority of this disease has increased significantly.6 Our statistics of EM-related ovarian clear cell carcinoma cases reported in the past 20 years show that most are Asian cases. Matalliotakis found that the incidence of ovarian endometriosis cysts in the perimenopausal group was higher than in the postmenopausal group (Perimenopausal group (125/184, 68%) vs Postmenopausal group (5/46, 10.8%), P<0.001).3 The risk of ovarian cancer in EMs women was 50% higher than in healthy women, and the risk of developing CCC was three times higher in healthy women.7
Inflammation of epithelial cell derivatives in the female genital tract is the leading cause of malignant transformation of endometriosis-related CCC.8 Relevant studies have shown that the early events (tumorigenesis) of EMs-related clear cell carcinoma are related to PI3K/AKT signal pathway activated by PIK3CA gene mutation.2 The KRAS gene mutation can cause cancer and is closely associated with EM-related endometrioid adenocarcinoma and clear cell carcinoma. It is involved in the late event (tumor growth) of EMs-related clear cell carcinoma by activating the RAS/ERK signal pathway.9 In the malignant transformation of EMs, atypical EMs are generally considered in the intermediate transition stage. Histologically, atypical endometriotic cells are highly similar to malignant ones.10 Atypical endometriosis is a precancerous lesion of CCC.7 Atypical EMs have increased endometrial glands, tight cell arrangement, cytoplasmic reduction, enlarged and moderately irregular nuclei, and no inflammatory cell infiltration. Malignant EMs are characterized by a significant increase in endometrial glands, disturbance of cell arrangement, cytoplasmic reduction, nuclear concentration, irregular shape and size, and infiltration of inflammatory cells.11 According to hematoxylin and eosin staining section, the two tumor cases mainly comprised atypical cells with clear cytoplasm, deep atomic staining, and enlarged nucleolus. In some areas, atypical cells with eosinophilic cytoplasm are mixed with spike-like atypical cells.
Concerning the location of ovarian cancer, research shows that the left side is the majority in perimenopause, and there is no significant difference after menopause.3 Through our analysis and summary of clear cell cancer case reports in the recent 20 years, we found that the incidence rate of ovarian cancer on the left side is higher than that on the right (52% vs 29%) (Table 1). This result is consistent with the above. According to the patient’s medical history, we can see that both patients had undergone endometriosis resection and received standard GnRH-A 6-needle treatment after surgery. Unfortunately, the cysts recurred in both patients. Although the recurrence rate of endometriosis has been estimated to reach 20% after two years and 40%-50% after five years,12 the malignant transformation rate after recurrence has not been reported. We raise the question of whether cyst recurrence contributes to the malignant transformation of endometriosis, which awaits more extensive data statistics and is essential in preventing the later malignant transformation of endometriosis.
|
Table 1 Characteristics of the Reported Cases of Endometriosis-Associated Clear Cell Carcinoma of the Ovary |
Surgical treatments aim to excise or ablate all visible diseases.30 When we look at the surgical methods of patients with ovarian clear cell carcinoma in the past 20 years, we find that radical surgery is the first choice (12/17, 70%) and postoperative adjuvant chemotherapy has gradually become the routine treatment. Of course, the five-year survival rate improved. With the trend of tumors in younger ages, in young women with low tumor stage and fertility requirements, we use fertility-preserving surgical treatment, such as cyst removal on the affected side. (Table 1) Although in patients undergoing complete surgical staging surgery, progression-free survival did not differ between intraoperative rupture and unrupture, regardless of the presence or absence of adjuvant platinum-based chemotherapy. In a recent large study, the risk associated with intraoperative rupture ovarian cancer depended on tissue type. It was most significant in patients with clear cell cancer and should be related to adhesions.31 Therefore, postoperative follow-up is essential. When found early, ovarian clear cell carcinoma is associated with a relatively good prognosis. Surgical treatment is the primary treatment, and adjuvant chemotherapy becomes the norm. For women with CCC, 35% of the patients have a complete clinical response to chemotherapy.32 Both patients have finally received six carboplatin and paclitaxel adjuvant chemotherapy courses. The postoperative survival rate of case 2 has reached six years, and there is no apparent recurrence probability. And the postoperative survival rate of case 1 has reached one year. So far, there is no obvious sign of recurrence. There is no significant difference in the prognosis of patients with CCC and serous adenocarcinoma in the early stage (I and II). Still, the recurrence rate of CCC is higher than that of serous adenocarcinoma. Close postoperative follow-up is necessary.
According to immunohistochemistry (IHC), the two patients were typical CCC. Estrogen receptor (ER) and progesterone receptor (PR) findings were negative. At the same time, CK7 and CA125 lesions are highly expressed, which is the IHC feature of typical CCC and strongly supports the histological results. Ki-67 is an excellent marker for determining the growth fraction of a given cell population, especially for evaluating cancer growth.33 Multivariate analysis showed that the Ki-67 labeling index was an independent prognostic factor for CCC. The Ki67 immunomarker reflects cell proliferation and spreading activity associated with severe adhesion34 and endometriotic cysts’ size.31 Some articles have summarized that low tumor proliferation may be a behavior of CCC resistance to chemotherapy.35 But some articles show that the epithelial-mesenchymal transition pathway may be central to chemotherapy resistance.36 The different expressions of Ki67 may be a manifestation of the difference in prognosis, and active follow-up is needed.
According to V2.2023 NCCN guidelines, Patients with pelvic mass or ascites, abdominal distension, and other obvious malignant-related symptoms underwent ultrasound (US) or abdominal/pelvic computed tomography (CT), magnetic resonance imaging (MRI), or PET-CT after necessary abdominal/pelvic examination, which mainly used for the initial stage of ovarian cancer. We found that the US can preliminarily determine the tumor’s location, boundary, and blood flow signal. Pelvic MRI can further show the origin of cancer and its relationship with surrounding tissues and show that it has a great application prospect in diagnosing malignant transformation of endometriosis. The current case study shows that preoperative MR relaxation measurement may be valuable for distinguishing EAOC and benign OE.25 R2 value can guide patients to choose before conservative treatment (including fertility-preserving surgery) and can be an effective parameter for EAOC diagnosis. For EMs patients of childbearing age or perimenopause, the R2 prediction index helps predict the malignant change of EMs.37 CT is often used for staging. Compared with CT, PET-CT has a higher diagnostic performance. A recent study of the Radiotracer 18F-Fluciclovine PET/CT showed that the sensitivity of this tracer in ovarian cancer patients was 100% (41/41).38 As we all know, a standardized uptake value (SUV) greater than 2.5 was considered a malignant tumor, and less than 10 suggested a good prognosis. Further studies should also focus on PET/CT scans to determine the best treatment for ovarian cancer, especially when malignancy is suspected.39 With the development of clinical practice towards precision medicine, it is essential to improve the accuracy of ovarian cancer staging and re-stage by evaluating the application of molecular imaging for different biological pathways in ovarian cancer.
Patient Perspective
Case 1: The operation was successful. There were some side effects during the chemotherapy, but I survived the whole chemotherapy phase smoothly, and now the tumor has not seen significant recurrence. I am glad that I have been taking physical examinations.
Case 2: I am recovering very well now. I have not had a relapse for so long. When you find a problem in the body, you must treat it early to get a good result.
Conclusion
The common OCCC in patients with endometriosis has obvious clinical signs. It is necessary to comprehensively evaluate whether there are risk factors for malignant transformation in combination with the patient’s medical history, tumor markers, imaging examination, and other factors. Surgical treatment is recommended for an excellent clinical outcome if the risk of malignant transformation is high. At the same time, to improve the survival and prognosis of CCC patients, further understanding the molecular mechanism of malignant transformation of endometriosis and exploring new treatment strategies, including molecular targets, is necessary.
Data Sharing Statement
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author.
Ethics
Institutional approval is required for the release of case details. The Second Affiliated Hospital of Zhejiang Chinese Medical University (Xinhua Hospital of Zhejiang Province) has approved the release of case details.
Informed Consent
Informed consent for case publication was obtained from the study participants. The patient provided written informed permission to publish case details and any accompanying images.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Mikhaleva LM, Davydov AI, Patsap OI, et al. Malignant transformation and associated biomarkers of ovarian endometriosis: a narrative review. Adv Ther. 2020;37(6):2580–2603. doi:10.1007/s12325-020-01363-5
2. Li B, Wang Y, Wang Y, et al. Deep infiltrating endometriosis malignant invasion of cervical wall and rectal wall with lynch syndrome: a rare case report and review of literature. Front Oncol. 2022;12:832228. doi:10.3389/fonc.2022.832228
3. Matalliotakis M, Matalliotaki C, Trivli A, et al. Keeping an eye on perimenopausal and postmenopausal endometriosis. Diseases. 2019;7(29):1–7. doi:10.3390/diseases7010029
4. Marie-Scemama L, Even M, Joliniere JBDL, et al. Endometriosis and the menopause: why the question merits our full attention. Horm Mol Biol Clin Investig. 2019;37(2):20180071. doi:10.1515/hmbci-2018-0071
5. Murakami K, Kotani Y, Shiro R, et al. Endometriosis-associated ovarian cancer occurs early during follow-up of endometrial cysts. Int J Clin Oncol. 2020;25:51–58. doi:10.1007/s10147-019-01536-5
6. Olivera K, Bradyb W, Birrer M, et al. An evaluation of progression free survival and overall survival of ovarian cancer patients with clear cell carcinoma versus serous carcinoma treated with platinum therapy: an NRG oncology/gynecologic oncology group experience. Gynecol Oncol. 2017;147(2):243–249. doi:10.1016/j.ygyno.2017.08.004
7. Sun Y, Liu G. Endometriosis-associated ovarian clear cell carcinoma: a special entity? J Cancer. 2021;12(22):6773–6786. PMCID: PMCPMC8518018. doi:10.7150/jca.61107
8. Chandler RL, Damrauer JS, Raab JR, et al. Coexistent ARID1A-PIK3CA mutations promote ovarian clear-cell tumorigenesis through pro-tumorigenic inflammatory cytokine signalling. Nat Commun. 2015;6:6118. doi:10.1038/ncomms7118
9. Teasley HE, Beesley A, Kim TH, et al. Differential expression of KRAS and SIRT1 in ovarian cancers with and without endometriosis. Reprod Sci. 2020;27(1):145–151. PMID: 32046380. doi:10.1007/s43032-019-00017-4
10. Streuli IGH, Wenger JM, Petignat P, Petignat P. Endometriosis after menopause: physiopathology and management of an uncommon condition. Climacteric. 2017;20(2):138–143. doi:10.1080/13697137.2017.1284781
11. Wang CT, Wang DB, Liu KR, et al. Inducing malignant transformation of endometriosis in rats by long-term sustaining hyperestrogenemia and type II diabetes. Cancer Sci. 2015;106(1):43–50. PMID: 25421527; PMCID: PMCPMC4317770. doi:10.1111/cas.12573
12. Horne AW, Saunders PTK. SnapShot: endometriosis. Cell. 2019;179(7):1677- e1. PMID: 31951524. doi:10.1016/j.cell.2019.11.033
13. Makrydimas G, Sotiriadis A, Paraskevaidis E, et al. Clear cell ovarian carcinoma in a pregnant woman with a history of infertility, endometriosis and unsuccessful IVF treatment. Eur J Gynaecol Oncol. 2003;24(5):438–441.
14. Saylam K, Devreker F, Simon P, et al. Ovarian clear cell carcinoma occurring in a young patient with endometriosis and long-term ovulation stimulations. Acta Obstet Gynecol Scand. 2006;85(12):1506–1507. doi:10.1080/00016340600603577
15. Murta EF, Nomelini RS, Ferreira FA, et al. Ovarian clear cell carcinoma associated with endometriosis: a case report with immunohistochemical study. Eur J Gynaecol Oncol. 2007;28(5):403–405.
16. Matsuo K, Alonsozana EL, Eno ML, et al. Primary peritoneal clear cell adenocarcinoma arising in previous abdominal scar for endometriosis surgery. Arch Gynecol Obstet. 2009;280(4):637–641. PMID: 19219617. doi:10.1007/s00404-009-0962-y
17. Fujiu K, Miyamoto H, Hashimoto S, et al. A case of diaphragmatic clear cell carcinoma in a patient with a medical history of ovarian endometriosis. Int J Clin Oncol. 2010;15(5):489–492. doi:10.1007/s10147-010-0052-y
18. Pergialiotis V, Lagkadas A, Polychronis O, et al.; Endometriosis-associated ovarian cancer. Presentation of a case report and review of the literature. Eur J Gynaecol Oncol. 2011;32(6):682–685. PMID: 22335037.
19. Shang HS, Chao TK, Wu GZ, et al. A rare case of the coexistence of ovarian clear cell carcinoma, mucinous cystadenoma, and endometriosis in the same ovary. Eur J Gynaecol Oncol. 2011;32(6):677–679.
20. Takahashi Y, Mogami H, Hamada S, et al. Alpha-fetoprotein producing ovarian clear cell carcinoma with a neometaplasia to hepatoid carcinoma arising from endometriosis: a case report. J Obstet Gynaecol Res. 2011;37(12):1842–1846. doi:10.1111/j.1447-0756.2011.01622.x
21. Win TT, Nik Mahmood NMZ, Ma SO, et al. Bilateral ovarian clear cell carcinoma arising in 17 year longstanding history of bilateral ovarian endometriosis. Iran J Pathol. 2016;11(5):478–482.
22. Zhou ZN, Tierney C, Rodgers WH, et al. Ruptured clear cell carcinoma of the ovary presenting as acute abdomen. Gynecol Oncol Rep. 2016;16:1–4. doi:10.1016/j.gore.2016.01.003
23. Komiyama S, Nagashima M, Taniguchi T, et al. Ovarian clear cell carcinoma detected during long-term management of endometriotic cysts in young patients: possible heterogeneity of this tumor. Gynecol Obstet Invest. 2019;84(3):305–312. doi:10.1159/000494256
24. Uehara T, Yoshida H, Tate K, et al. Metachronous occurrence of two different histological subtypes of endometriosis-related neoplasms. Gynecol Oncol Rep. 2019;27:42–45. doi:10.1016/j.gore.2018.12.007
25. Matsubara S, Kawahara N, Horie A, et al. Magnetic resonance relaxometry improves the accuracy of conventional MRI in the diagnosis of endometriosis-associated ovarian cancer: a case report. Mol Clin Oncol. 2019;11(3):296–300. doi:10.3892/mco.2019.1889
26. Inamdar A, Fulginiti A, Yiu SF, et al. Metastatic ovarian clear cell carcinoma in the context of in vitro fertilization pregnancy. Case Rep Obstet Gynecol. 2020;2020:2695058. doi:10.1155/2020/2695058
27. Negrão E, Flor-de-lima B, Duarte AL, et al. Ovarian clear cell carcinoma arising in a large endometrioma – A case report with pathological correlation and literature review. Radiol Case Rep. 2022;17(8):2806–2811. doi:10.1016/j.radcr.2022.05.015
28. Mendoza Stanteen S, Pak T, Chen H, et al. Clear cell carcinoma arising from ovarian and thoracic endometriosis: a case report and review of literature. Case Rep Obstet Gynecol. 2022;2022:7624305. doi:10.1155/2022/7624305
29. Farah AM, Gu S, Jia Y. Clinical analysis and literature review of a case of ovarian clear cell carcinoma with PIK3CA gene mutation: a case report. Medicine. 2022;101(37):e30666. PMCID: PMCPMC9478318. doi:10.1097/MD.0000000000030666
30. Hermens M, van Altena AM, Nieboer TE, et al. Incidence of endometrioid and clear-cell ovarian cancer in histological proven endometriosis: the ENOCA population-based cohort study. Am J Obstet Gynecol. 2020;223(1):107 e1- e11. doi:10.1016/j.ajog.2020.01.041
31. Min KW, Park MH, Hong SR, et al. Clear cell carcinomas of the ovary: a multi-institutional study of 129 cases in Korea with prognostic significance of Emi1 and Galectin-3. Int J Gynecol Pathol. 2013;32(1):3–14. PMID: 23202783. doi:10.1097/PGP.0b013e31825554e9
32. Ho CM, Huang YJ, Chen TC, et al. Pure-type clear cell carcinoma of the ovary as a distinct histological type and improved survival in patients treated with paclitaxel-platinum-based chemotherapy in pure-type advanced disease. Gynecol Oncol. 2004;94(1):197–203. doi:10.1016/j.ygyno.2004.04.004
33. Bartiromo L, Schimberni M, Villanacci R, et al. A systematic review of atypical endometriosis-associated biomarkers. Int J Mol Sci. 2022;23(8):4425. PMID: 35457244; PMCID: PMCPMC9029517. doi:10.3390/ijms23084425
34. Penciu RC, Postolache I, Steriu L, et al. Is there a relationship in-between ovarian endometriosis and ovarian cancer? Immunohistochemical profile of four cases with coexisting ovarian endometriosis and cancer. Rom J Morphol Embryol. 2020;61(1):157–165. PMID: 32747907; PMCID: PMCPMC7728120. doi:10.47162/RJME.61.1.18
35. Itamochi H, Kigawa J, Terakawa N. Mechanisms of chemoresistance and poor prognosis in ovarian clear cell carcinoma. Cancer Sci. 2008;99(4):653–658. PMID: 18377417. doi:10.1111/j.1349-7006.2008.00747.x
36. Takahashi N, Hatakeyama K, Nagashima T, et al. Characterization of rare histological subtypes of ovarian cancer based on molecular profiling. Cancer Med. 2023;12(1):387–395. PMID: 35676859; PMCID: PMCPMC9844652. doi:10.1002/cam4.4927
37. Yamanaka S, Kawahara N, Kawaguchi R, et al. The comparison of three predictive indexes to discriminate malignant ovarian tumors from benign ovarian endometrioma: the characteristics and efficacy. Diagnostics. 2022;12:1212. doi:10.3390/DIAGNOSTICS12051212
38. Buehner TM, Liotta M, Potkul RK, et al. Initial experience with the radiotracer 18F-fluciclovine PET/CT in ovarian cancer. Mol Imaging Biol. 2023. PMID: 36754935. doi:10.1007/s11307-023-01807-8
39. American Cancer Society. Key statistics for ovarian cancer. American Cancer Society; 2023. Available from: https://www.cancer.org/cancer/ovarian-cancer/about/key-statistics.html. Accessed October 16, 2023.