Congenital disorders
Incidence and risk factors for retinopathy of prematurity
Introduction
Retinopathy of prematurity (ROP), a vasoproliferative disorder of the immature retina, is currently one of the main causes of blindness or visual impairment of preterm infants, also related to long-term neurological and cognitive development disorders.1 Recent research showed that with the increase of the survival rate of preterm infants, especially very low birth weight (VLBW) infants, the incidence of ROP has gradually increased in both developed and developing countries.2–4 ROP is a kind of multifactorial disease. Small GA, low BW, and prolonged oxygen therapy are all risk factors for the development of ROP.5,6 Some studies also showed that gender and multiple pregnancies were risk factors for ROP.7,8
The occurrence rate of ROP in China varies significantly based on the region and the level of medical care.9,10 This study aimed to analyze the incidence and risk factors of ROP in preterm infants with BW <1500 grams in our NICU and increase the awareness of neonatologists in preventing ROP in clinical work.
Methods
Population
The clinical data of preterm infants who were admitted to the Department of Neonatology, Obstetrics and Gynecology Hospital of Affiliated to Nanjing Medical University from January 2019 to December 2022 was collected. Inclusion criteria: birth weight (BW) <1500g. Exclusion criteria: 1. Died or transferred to other hospitals before ROP screening; 2. Not do ROP screening or missing data; 3. Severe congenital developmental abnormalities.
This study has been approved by the Ethics Committee of Obstetrics and Gynecology Hospital of Affiliated to Nanjing Medical University, and all procedures were performed according to the tenets of the Declaration of Helsinki. Written informed consent for data collection was obtained from the parent/guardian of each child.
ROP Screening
In our NICU, all ROP screenings were performed by ophthalmologists at our hospital, and regular follow-up is conducted at the ophthalmology department of our hospital after patients’ discharge.
Eye examinations were performed on all preterm neonates who met one of the following criteria: GA≤ 34w or BW≤ 2000g; This range may be extended for patients with a long history of oxygen inhalation, severe illnesses like severe sepsis, or persistent pulmonary hypertension of newborns (PPHN). The initial time for ROP screening refers to the American Academy of Pediatrics (AAP) recommendation.11 In this study, severe ROP was defined as stage 3, presence of plus disease, or needing treatment.
Statistical Analysis
All analyses were performed using SPSS (version 26.0). The non-normal distributed continuous variables were shown as medians with interquartile ranges [M(P25, P75)] and analyzed using the Mann–Whitney test. Categorical variables were shown as rates and analyzed using the Chi-squared test. The variables with statistically significant differences selected from the univariate analysis were included in the multivariate logistic regression analysis, and odds ratios (OR) were calculated. For all tests, a P-value <0.05 was considered statistically significant.
Results
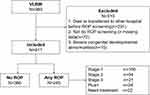
A total of 611 preterm infants were included in this study. The screening process is shown in Figure 1. The number of infants with any stage ROP was 245(40.1%). Among them, 160(26.2%) infants were stage 1, 54(8.8%) were stage 2, and 31(5.1%) were stage 3, no stage 4 and 5, 22(3.6%) infants needed treatment for ROP.
|
Figure 1 Flowchart of the study population. Abbreviations: VLBW, very low birth weight infant; ROP, retinopathy of prematurity. |
The perinatal characteristics are shown in Table 1 and the clinical characteristics in Table 2. Univariate analysis showed that infants in both any stage ROP group and the severe ROP group had significantly lower GA and BW, a higher rate of serious complications, including moderate-to-severe BPD, grade III~IV intraventricular hemorrhage (IVH), culture-positive sepsis. These infants also needed more days of total oxygen, non-invasive and invasive respiratory support, and more numbers of red blood cell (RBC) transfusion during hospitalization in the NICU. The difference is that the rate of male gender is higher in severe group and twin birth in any stage ROP group.
|
Table 1 Perinatal Characteristics |
 |
Table 2 Clinical Characteristics |
Multivariate logistic regression analysis showed that a higher BW (P < 0.02, OR: 0.995, 95% CI: 0.992–0.998) was protective, whereas male gender (P = 0.026, OR: 2.574 95% CI: 1.118–5.926) was the risk factor for severe ROP (Table 3). A higher GA (P < 0.01, OR: 0.718, 95% CI: 0.593–0.871) was protective, whereas twin birth (P = 0.026, OR: 1.594, 95% CI: 1.056–2.406) and moderate-to-severe BPD (P = 0.016, OR: 2.334, 95% CI: 1.147–4.625) increased the hazard of any stage ROP (Table 4).
 |
Table 3 Multivariate Analysis of Risk Factors for Severe ROP |
 |
Table 4 Multivariate Analysis of Risk Factors for Any Stage ROP |
Discussion
Our study revealed the incidence and risk factors of any stage and severe ROP in premature infants with BW <1500g. As one of the serious complications of preterm infants, the incidence of ROP also tends to increase as the survival rate of these infants gradually increases.12 The incidence of any stage ROP in our NICU was 40.1%, located in the upper part of the range from 18.5% to 47%, as reported.13–15 This result may be related to the difference between levels of care in NICUs, ROP screening methods, and study populations. For the rate of ROP needed treatment, our NICU was lower than some others.16,17
As is well known, smaller GA and lower BW are the two strongest known risk factors for the development of ROP.18,19 The main reason for this is that preterm infants with smaller GA and lower BW have more immature retinas and a higher opportunity to be exposed to the risk factors of ROP, such as high-concentration oxygen inhalation and infection.20 In our study, we found that smaller GA was the risk factor of any stage ROP but not the severe ROP, lower BW was the risk factor of severe ROP but not the any stage ROP.
Male gender was a risk factor for severe ROP, as shown in our study. This result was the same as the studies of Yang’s and Mantapond’s.7,8 Other studies had also reported this result.21,22 A study showed that male fetal sex was associated with higher maternal levels of pro-inflammatory cytokines and angiogenic factors including VEGF during pregnancy, suggesting potential harmful effects on development of ROP in male infants.23 However, the relationship between gender and ROP remains controversial as some studies found no difference in the incidence of ROP by gender.24
For any stage ROP, twin birth and moderate-to-severe BPD were risk factors besides smaller GA. Multiple gestation is associated with increased risk for preterm birth, smaller BW, and perinatal morbidities, which may affect ROP risk.25 Mantapond and coworkers reported that multiple birth has been associated with treatment-requiring ROP, but this condition was not associated with any ROP development.8 Our research results differ from this, possibly due to differences in inclusion criteria. There are also several studies reported that there were no differences between singleton and multiple births.26,27 So more studied are needed to prove the relationship between multiple gestation and ROP.
Moderate-to-severe BPD was one of the risk factors for any stage of ROP in our study, the same result as many other studies.28,29 Stark and coworkers reported that both BPD and ROP may share common molecular mechanisms predisposing to dysregulation of angiogenesis.30 Some studies suggested that corticosteroids play an important role in the relationship between the BPD and ROP.31
One limitation of our study is that many patients were lost to follow up or died after the first time screening, so we could not know if their ROP had progressed. It is difficult to avoid selection bias and recall bias for a case–control study. The strength of our study is for a single-center study, we have a larger number of cases.
Conclusion
The incidence of ROP at any stage and ROP requiring treatment was 40.1% and 3.6% respectively in our NICU. Higher BW was protective, whereas male gender were risk factors for the development of severe ROP. A higher GA was protective, whereas twin birth and moderate-to-severe BPD increased the hazard of any stage ROP.
Abbreviations
BW, birth weight; GA, gestational age; NICU, neonatal intensive care unit; PMA, post-menstrual age; ROP, retinopathy of prematurity; GDM, gestational diabetes mellitus; BPD, Bronchopulmonary dysplasia; NEC, Necrotizing enterocolitis; IVH, Intraventricular hemorrhage; RBC, red blood cell.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Funding
This study was supported by Key talents project of maternal and child health in Jiangsu province.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Molloy CS, Anderson PJ, Anderson VA, et al. The long-term outcome of extremely preterm (<28 weeks’ gestational age) infants with and without severe retinopathy of prematurity. J Neuropsychol. 2016;10(2):276–294. doi:10.1111/jnp.12069
2. Austeng D, Källen K, Hellström A, et al. Regional differences in screening for retinopathy of prematurity in infants born before 27 weeks of gestation in Sweden—the EXPRESS study. Acta Ophthalmol. 2014;92(4):311–315. doi:10.1111/aos.12165
3. Xu Y, Zhou X, Zhang Q, et al. Screening for retinopathy of prematurity in China: a neonatal units-based prospective study. Invest Ophthalmol Vis Sci. 2013;54(13):8229–8236. doi:10.1167/iovs.13-12297
4. Cerman E, Balci SY, Yenice OS, et al. Screening for retinopathy of prematurity in a tertiary ophthalmology department in Turkey: incidence, outcomes, and risk factors. Ophthalmic Surg Lasers Imaging Retina. 2014;45(6):550–555. doi:10.3928/23258160-20141118-10
5. Sang JK, Alexander DP, Ryan S, et al. Retinopathy of prematurity: a review of risk factors and their clinical significance. Surv Ophthalmol. 2018;63(5):618–637. doi:10.1016/j.survophthal.2018.04.002
6. Ann H, Lois S, Olaf D. Retinopathy of prematurity. Lancet. 2013;382(9902):1445–1457. doi:10.1016/S0140-6736(13)60178-6
7. Michael BY, Edward FD, Jordan RW. Race, gender, and clinical risk index for babies (CRIB) score as predictors of severe retinopathy of prematurity. J AAPOS. 2006;10(3):253–261. doi:10.1016/j.jaapos.2006.01.004
8. Mantapond I, Supakorn C, Sunee C. Incidence and risk factors for retinopathy of prematurity at a Rural Tertiary Hospital in Thailand. J Ophthalmic Vis Res. 2023;18(1):81–87. doi:10.18502/jovr.v18i1.12728
9. Li L, Yanlin G, Wei C, Han M. Screening for retinopathy of prematurity in North China. BMC Ophthalmol. 2022;22(1):251. doi:10.1186/s12886-022-02470-3
10. Yi D, Li Z, Yequn Z. Incidence of retinopathy of prematurity treatment in extremely preterm infants in China. Paediatr Perinat Epidemiol. 2022;36(3):380–389. doi:10.1111/ppe.12810
11. Fierson WM, Chiang MF, Good W; American Academy of Pediatrics Section on Ophthalmology; American Academy of Ophthalmology; American Association for Pediatric Ophthalmology and Strabismus; American Association of Certified Orthoptists. Screening examination of premature infants for retinopathy of prematurity. Pediatrics. 2018;142(6):e20183061. doi:10.1542/peds.2018-3061
12. Shah PK, Prabhu V, Karandikar SS, et al. Retinopathy of prematurity: past, present and future. World J Clin Pediatr. 2016;5(1):35–46. doi:10.5409/wjcp.v5.i1.35
13. Bowe T, Nyamai L, Ademola-Popoola D, et al. The current state of retinopathy of prematurity in India, Kenya, Mexico, Nigeria, Philippines, Romania, Thailand, and Venezuela. Digit J Ophthalmol. 2019;25(4):49–58. doi:10.5693/djo.01.2019.08.002
14. Shah VA, Yeo CL, Ling YL, Ho LY. Incidence, risk factors of retinopathy of prematurity among very low birth weight infants in Singapore. Ann Acad Med Singap. 2005;34(2):169–178. PMID: 15827664.
15. Yau GS, Lee JW, Tam VT, et al. Incidence and risk factors of retinopathy of prematurity from 2 neonatal intensive care units in a Hong Kong Chinese population. Asia Pac J Ophthalmol. 2016;5(3):185–191. doi:10.1097/APO.0000000000000167
16. Ahmet YB, Nihal D, Esin K, et al. Incidence, risk factors and severity of retinopathy of prematurity in Turkey (TR-ROP study): a prospective, multicentre study in 69 neonatal intensive care units. Br J Ophthalmol. 2018;102(12):1711–1716. doi:10.1136/bjophthalmol-2017-311789
17. Gillian GA, Catey B, Wen X, et al. Retinopathy of prematurity in the United Kingdom: retreatment rates, visual and structural 1-year outcomes. Eye. 2018;32(11):1752–1759. doi:10.1038/s41433-018-0151-y
18. Carlo D, Caterina C, Fiorenza P, et al. Incidence and risk factors of retinopathy of prematurity in an Italian cohort of preterm infants. Ital J Pediatr. 2021;47(1):64. doi:10.1186/s13052-021-01011-w
19. Ali AA, Gomaa NAS, Awadein AR, et al. Retrospective cohort study shows that the risks for retinopathy of prematurity included birth age and weight, medical conditions and treatment. Acta Paediatr. 2017;106(12):1919–1927. doi:10.1111/apa.14019
20. Pia L, Anna K, Eva MA, et al. Low birth weight is a risk factor for severe retinopathy of prematurity depending on gestational age. PLoS One. 2014;9(10):e109460. doi:10.1371/journal.pone.0109460
21. Slidsborg C, Jensen A, Forman JL, et al. Neonatal risk factors for treatment-demanding retinopathy of prematurity: a Danish National Study. Ophthalmology. 2016;123(4):796–803. doi:10.1016/j.ophtha.2015.12.019
22. Arlette JS, Jacqueline UT, Frank TK, et al. Nationwide inventory of risk factors for retinopathy of prematurity in the Netherlands. J Pediatr. 2014;164(3):494–498.e1. doi:10.1016/j.jpeds.2013.11.015
23. Enninga EA, Nevala WK, Creedon DJ, et al. Fetal sex-based differences in maternal hormones, angiogenic factors, and immune mediators during pregnancy and the postpartum period. Am J Reprod Immunol. 2015;73(3):251–262. doi:10.1111/aji.12303
24. Chiang MF, Arons RR, Flynn JT, et al. Incidence of retinopathy of prematurity from 1996 to 2000: analysis of a comprehensive New York state patient database. Ophthalmology. 2004;111(7):1317–1325. doi:10.1016/j.ophtha.2003.10.030
25. The ESHRE Capri Workshop Group. Multiple gestation pregnancy. Hum Reprod. 2000;15(8):1856–1864. PMID: 10920117. doi:10.1093/humrep/15.8.1856
26. Blumenfeld LC, Siatkowski RM, Johnson RA, et al. Retinopathy of prematurity in multiple gestation pregnancies. Am J Ophthalmol. 1998;125(2):197–203. doi:10.1016/s0002-9394(99)80092-0
27. Friling R, Rosen SD, Monos T, et al. Retinopathy of prematurity in multiple-gestation, very low birth weight infants. J Pediatr Ophthalmol Strabismus. 1997;34(2):96–100. doi:10.3928/0191-3913-19970301-08
28. Gebesce A, Uslu H, Keles E, et al. Retinopathy of prematurity: incidence, risk factors, and evaluation of screening criteria. Turk J Med Sci. 2016;46(2):315–320. doi:10.3906/sag-1407-127
29. Port AD, Chan RV, Ostmo S, et al. Risk factors for retinopathy of prematurity: insights from outlier infants. Graefes Arch Clin Exp Ophthalmol. 2014;252(10):1669–1677. doi:10.1007/s00417-014-2716-1
30. Stark A, Dammann C, Nielsen HC, Volpe MV. A pathogenic relationship of bronchopulmonary dysplasia and retinopathy of prematurity? A review of angiogenic mediators in both diseases. Front Pediatr. 2018;6:125. doi:10.3389/fped.2018.00125
31. Liu P-M, Fang P-C, Huang C-B, et al. Risk factors of retinopathy of prematurity in premature infants weighing less than 1600 g. Am J Perinatol. 2005;22(2):115–120. doi:10.1055/s-2005-837276