Infection
Mycobacterium abscessus scalp infection after hair transplantation in Central America
KEY POINTS
-
Mycobacterium abscessus is an uncommon cause of iatrogenic infections.
-
M. abscessus (subspecies massiliense) is a nontuberculous mycobacteria that contains a nonfunctional erm gene, conferring macrolide susceptibility.
-
The treatment of cutaneous infections caused by M. abscessus uses a combination of surgical débridement and several antimicrobial agents.
A 53-year-old previously healthy man presented with painful, red nodules on his anterior scalp 3 weeks after a hair transplantation in Panama. The patient had about 6000 single hair follicles manually inserted into the superior and anterior regions of his scalp during an 8-hour surgical procedure using the direct hair implantation technique. The procedure was well tolerated and the patient returned to North America. Nineteen days after hair transplantation, the patient noticed nodules on the anterior scalp that gradually increased in size over 4 days, along with erythema, pain and tenderness along the scalp and spontaneous purulent drainage. He was seen by his family physician and prescribed 6 days of oral doxycycline (100 mg, twice daily), followed by oral cephalexin (500 mg, 4 times daily). He continued to have spontaneous purulent drainage despite 7 days of antimicrobial therapy and presented to our emergency department 1 day after starting cephalexin.
On presentation the patient was afebrile and showed no systemic signs of infection. Over the anterior scalp, he had a region of fluctuance packed with surgical gauze, with purulent drainage and surrounding erythema. The donor hair site at the posterior scalp appeared normal. He had a normal leukocyte count and normal levels of serum creatinine, electrolytes and C-reactive protein. In the emergency department, needle aspiration was performed and a deep-tissue specimen was submitted for microbiological analysis. The emergency physician started 5 days of intravenous cefazolin (2 g/d), oral probenacid (1 g/d) and trimethoprim–sulfamethoxazole (1 double-strength oral tablet, twice daily) for 1 week. The infection did not resolve on this regimen.
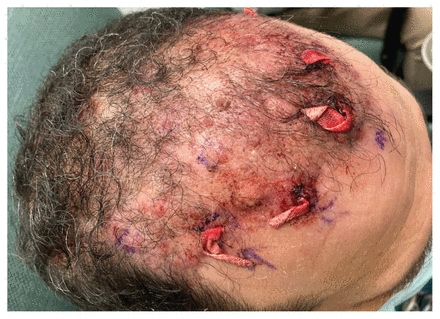
After 5 weeks of incubation, Mycobacterium abscessus (subspecies massiliense) was isolated from the deep-tissue specimen, with antimicrobial susceptibilities as reported in Table 1. The patient underwent incision and drainage of 5 abscesses of the anterior scalp about 2 months after hair transplantation (Figure 1). Based on the results of drug susceptibility testing, the infectious disease physician started the patient on combination antimicrobial therapy with intravenous amikacin (1500 mg [18 mg/kg], 3 times/wk) and oral clarithromycin (500 mg, twice daily). We followed the patient with weekly audiometry assessments and bloodwork. Therapeutic drug monitoring targeted peak amikacin levels of 65–80 mg/L and trough levels of less than 5 μg/mL.
Susceptibility results of Mycobacterium abscessus (subspecies massiliense) isolated from a man’s scalp abscesses after hair transplantation
” data-icon-position data-hide-link-title=”0″>
Scalp of a 53-year-old man with Mycobacterium abscessus infection after incision and drainage of scalp nodules (with quarter-inch ribbon packing strips in situ), 2 months after hair transplantation and before starting amikacin and clarithromycin.
One month after starting antimicrobial therapy, the patient noticed nocturnal tinnitus, and audiometry testing detected mild hearing loss in the right ear. By this time, he had substantial improvement in the erythema and swelling of the nodules (Figure 2). The patient also reported resolution of pain and tenderness to the respective area. We stopped amikacin and continued clarithromycin monotherapy for another 5 months. The patient’s tinnitus and hearing loss resolved after stopping amikacin and his nodules and abscesses were resolved completely at the end of therapy. About 3 months after completing clarithromycin therapy, he had no signs of relapsed infection.
” data-icon-position data-hide-link-title=”0″>

Scalp of a 53-year-old man with Mycobacterium abscessus infection after completing 1 month of combination therapy of amikacin and clarithromycin.
Discussion
Increasingly, patients seek medical treatment abroad, and reports of challenging postoperative surgical site infections are increasing. Our patient’s multidrug-resistant M. abscessus scalp infection after hair transplantation in Central America exemplifies the precautionary concern that is warranted for people who seek cosmetic procedures outside Canada. Although M. abscessus is prevalent throughout the world, the incidence of infection can vary.1 Regions such as East Asia and the islands in the south and central Pacific Ocean have been shown to have higher rates of M. abscessus infections.1,2 In British Columbia, the incidence of nontuberculous mycobacteria (NTM) pulmonary disease is lower than in other regions and is about 1.6 out of 100 000, with cutaneous infections being less common.2,3
Nontuberculous mycobacteria are found ubiquitously in the environment and cause 7 major clinical syndromes: pulmonary disease, lymphadenitis, skin and soft-tissue infection, skeletal infection, disseminated infection, catheter-related infection and hypersensitivity pneumonitis.4 The most common NTMs causing skin and soft-tissue infections are Mycobacterium chelonae, Mycobacterium fortuitum and M. abscessus.5
M. abscessus grows rapidly, is drug-resistant and can resist high temperatures and nutritionally deficient environments.4 It has been associated with contamination of water sources, hospital equipment and medications.6–10 It has also been described in complications following hair transplantation.11 The M. abscessus complex comprises 3 subspecies, namely M. abscessus subspecies abscessus, M. abscessus subspecies bolletii and M. abscessus subspecies massiliense. Infections caused by M. abscessus are often difficult to treat because they are intrinsically resistant to classic antituberculous medications and most classes of antibiotics. The presence of a functional erm(41) gene inactivates macrolides through inducible macrolide resistance. M. abscessus subspecies massiliense isolates do not contain the functional erm(41) gene and so are intrinsically susceptible to macrolide.12 These isolates respond well to clarithromycin-based regimens.
The clinical presentation of M. abscessus infection includes abscesses, nodules, cellulitis, panniculitis, ulcers and sinus drainage, often presenting weeks to months after the surgical procedure.13 Diagnosis is made by isolation of the organism from clinical specimens. Nontuberculous mycobacteria are difficult to identify on Gram stains and routine cultures. Combined with the slow turnaround time for antimicrobial susceptibility testing of NTMs, this difficulty often leads to a delay in diagnosis and starting appropriate antimicrobial therapy. The selection of antibiotics is typically guided by drug susceptibility results, given the complexity of antimicrobial resistance.13
A case series of 6 surgical site infections caused by NTMs in India reported response to treatment with clarithromycin monotherapy in 5 of 6 cases after 2 months, with 1 case in the shoulder joint requiring combination clarithromycin and amikacin. All 6 NTM isolates were susceptible to clarithromycin, and surgical débridement was not performed.14 Another case series reported 5 M. abscessus subspecies abscessus infections after cosmetic surgical procedures in Ecuador that were successfully treated with surgical débridement and combination antimicrobial therapy for 7 months.15 A third case series reported 10 surgical site infections caused by NTMs in Venezuela that were successfully treated with a combination of surgical débridement and prolonged combination antimicrobial therapy (> 3 mo), including clarithromycin.13
Clarithromycin monotherapy has been shown to be less efficacious than a combination of surgical intervention and antibiotic therapy.16 Furthermore, another study found that concomitant use of clarithromycin and amikacin may be associated with increased resolution of lesions when compared with monotherapy with either antibiotic in patients who developed a M. abscessus cutaneous infection after acupuncture.17 In a study comparing combination amikacin and clarithromycin or azithromycin to triple therapy with amikacin, either clarithromycin or azithromycin, and either cefoxitin or imipenem in patients with M. abscessus pulmonary disease, rates of treatment success and relapse were comparable. However, the triple-agent regimen was associated with an increased frequency of adverse reactions.18
Given the paucity of data on treatment of M. abscessus cutaneous infections, we extrapolated the use of susceptibility-based combination treatment for our patient with M. abscessus scalp infection. We treated him with a combination of surgical débridement and targeted antimicrobial therapy. We used both clarithromycin and amikacin, as well as close monitoring of audiometry and therapeutic drug levels. We opted for weekly audiometry monitoring because we anticipated a prolonged course of exposure to amikacin. The amikacin course was limited by tinnitus at 1 month, so we prescribed clarithromycin monotherapy for another 5 months.
With an increasing number of patients seeking medical treatment abroad, infectious complications pose a challenge for patients and health care systems. M. abscessus infections are an uncommon complication of cosmetic surgical procedures, but should be considered in patients with postoperative infections that do not respond to standard antimicrobial therapy. Microbiological specimens should be obtained to determine the organism and susceptibilities to antibiotics. Surgical drainage and débridement should be used in combination with antibiotics.
Footnotes
-
Competing interests: None declared.
-
This article has been peer reviewed.
-
The authors have obtained patient consent.
-
Contributors: All of the authors contributed to the conception and design of the work, drafted the manuscript, revised it critically for important intellectual content, gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use), and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/